15471-17-7
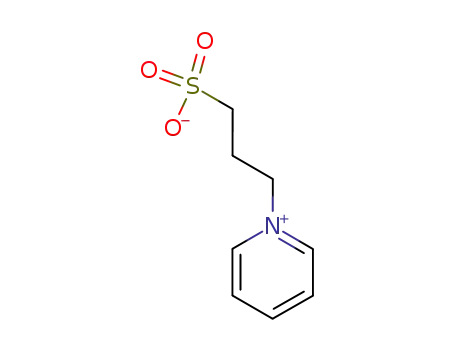
- Product Name:3-(1-Pyridinio)-1-propanesulfonate
- Molecular Formula:C8H11NO3S
- Purity:99%
- Molecular Weight:201.246
Product Details;
CasNo: 15471-17-7
Molecular Formula: C8H11NO3S
Appearance: white solid
Factory Supply Quality 3-(1-Pyridinio)-1-propanesulfonate 15471-17-7 with Efficient Transportation
- Molecular Formula:C8H11NO3S
- Molecular Weight:201.246
- Appearance/Colour:white solid
- Vapor Pressure:0Pa at 25℃
- Melting Point:275-277 °C
- Flash Point:160 °C
- PSA:87.18000
- Density:1.53[at 20℃]
- LogP:0.55470
3-(1-Pyridinio)-1-propanesulfonate(Cas 15471-17-7) Usage
|
Uses |
Pyridinium propyl sulfobetaine can be used: As a Bronsted acid ionic liquid catalyst in combination with H2SO4 and H3PO4 for the dehydration of glycerol to acrolein via semi-batch reaction technique. As a catalyst in the form of sulfonated heteropoly acid salts for the synthesis of isoamyl isovalerate. In the preparation of molybdenum imido alkylidene NHC catalysts, which are used in biphasic olefin metathesis. To prepare water-stable SO3H-functionalized Br?nsted acidic task-specific ionic liquids (TSILs). In was bright nickel plating, it is a start material to make brightener, A high efficiency brightener and leveler. Because of its high purity, Pyridinium propyl sulfobetaine cannot carry other harmful impurity or salts to baths. |
|
Description |
3-(1-Pyridinio)-1-propanesulfonate is a white crystalline powder used to prepare electroplating additives.It has good thermal qualitative and chemical stability, and its mechanical strength is also very good. The low polymer molecular weight is prone to disproportionation and cross-linking reactions. |
|
Chemical Properties |
3-(1-Pyridinio)-1-propanesulfonate is White Solid |
|
Application |
3-(1-Pyridinio)-1-propanesulfonate is used in the preparation of electroplating additives,and it is used in medicine and daily chemicals. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C8H11NO3S/c10-13(11,12)8-4-7-9-5-2-1-3-6-9/h1-3,5-6H,4,7-8H2
15471-17-7 Relevant articles
Reversible phase transformation gel-type ionic liquid compounds based on tungstovanadosilicates
Huang, Tianpei,Xie, Zhirong,Wu, Qingyin,Yan, Wenfu
, p. 17 - 22 (2016)
A series of new reversible phase transfo...
Heteropolyacid-based ionic liquids as effective catalysts for the synthesis of benzaldehyde glycol acetal
Han, Xiaoxiang,Yan, Wei,Chen, Keke,Hung, Chin-Te,Liu, Li-Li,Wu, Pei-Hao,Huang, Shing-Jong,Liu, Shang-Bin
, p. 149 - 156 (2014)
A series of environmental benign ionic l...
The synthesis and electrical properties of hybrid gel electrolytes derived from Keggin-type heteropoly acids and 3-(pyridin-1-ium-1-yl)propane-1-sulfonate (PyPs)
Narayanan, Sumaletha,Tong, Xia,Thangadurai, Venkataraman
, p. 102549 - 102556 (2016)
Herein, we report the effect of the prot...
Synthesis of 9,9-bis(4-hydroxyphenyl) fluorene catalyzed by bifunctional ionic liquids
Bai, Wei,Gao, Zhanxian,Lu, Xinxin,Wei, Jialun,Yan, Lei,Yu, Limei
, p. 32559 - 32564 (2021/12/07)
Through structural design, a series of b...
Dual targeting of cholinesterase and amyloid beta with pyridinium/isoquinolium derivatives
Chakravarty, Harapriya,Ju, Yaojun,Chen, Wen-Hua,Tam, Kin Y.
, p. 242 - 255 (2019/12/27)
With the surge in the cases of Alzheimer...
Microwave-promoted one-pot three-component synthesis of 2,3-dihydroquinazolin-4(1H)-ones catalyzed by heteropolyanion-based ionic liquids under solvent-free conditions
Yang, Yang,Fu, Renzhong,Liu, Yang,Cai, Jing,Zeng, Xiaojun
, (2020/06/09)
A series of 2,3-dihydroquinazoline-4(1H)...
Condensation of 9-fluorenone and phenol using an ionic liquid and a mercapto compound synergistic catalyst
Lei, Yan,Yu, Limei,Shen, Maochang,Luo, Shikang,Gao, Zhanxian
supporting information, p. 15700 - 15705 (2019/10/19)
A series of ionic liquids (ILs) were syn...
15471-17-7 Process route
-
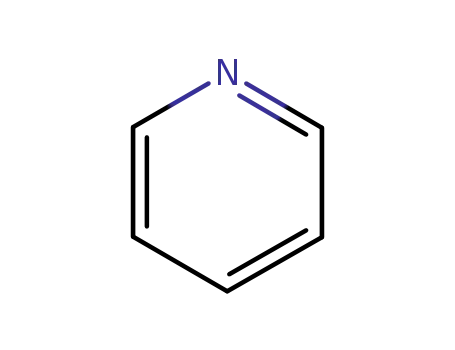
- 110-86-1
pyridine

-
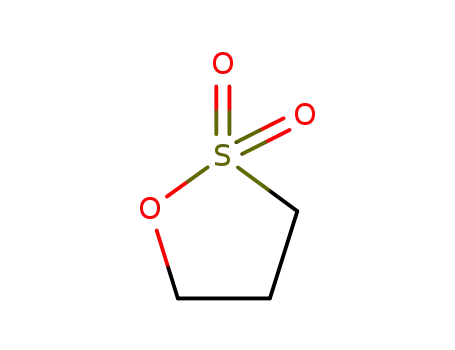
- 1120-71-4
1,3-propanesultone

-
- 15471-17-7
1-(3-sulfopropyl)pyridine
| Conditions | Yield |
|---|---|
|
for 1h; Ambient temperature;
|
98% |
|
for 0.00416667h; microvawe irradiation;
|
95% |
|
In ethyl acetate; at 60 ℃; for 2.5h;
|
86% |
|
In acetonitrile; at 70 ℃; for 24h;
|
85% |
|
|
|
|
In toluene; acetonitrile; at 50 ℃; for 2h; Cooling with ice;
|
|
|
In toluene; at 40 ℃; for 24h; Inert atmosphere;
|
|
|
In toluene; at 50 ℃; for 24h; Inert atmosphere;
|
|
|
at 80 ℃;
|
|
|
In toluene; at 50 ℃; for 24h; Inert atmosphere;
|
|
|
Reflux;
|
|
|
In toluene; at 50 ℃; for 24h; Inert atmosphere;
|
|
|
In toluene; at 50 ℃; for 24h; Inert atmosphere;
|
|
|
In acetone; at 50 ℃; for 4h;
|
|
|
In toluene; at 50 ℃; for 24h; Inert atmosphere;
|
|
|
at 40 ℃; for 24h; Inert atmosphere;
|
|
|
In acetone; at 50 ℃; for 4h;
|
|
|
at 40 ℃; for 24h;
|
|
|
In toluene; at 20 ℃; for 4h; Cooling with ice;
|
|
|
In ethanol; at 24.84 ℃;
|
|
|
In toluene; Inert atmosphere; Heating;
|
|
|
In toluene; at 50 ℃; for 24h; Inert atmosphere;
|
|
|
In ethyl acetate; at 90 ℃; for 12h;
|

-

- 15471-17-7
1-(3-sulfopropyl)pyridine
| Conditions | Yield |
|---|---|
|
Pyridin, 1,3-Propansulfon;
|
|
|
Pyridin, 3-Hydroxy-1-propansulfonsaeure;
|
|
|
Pyridin, Propansulton;
|
|
|
HO-
|
15471-17-7 Upstream products
-
110-86-1

pyridine
-
1120-71-4

1,3-propanesultone
Relevant Products
-
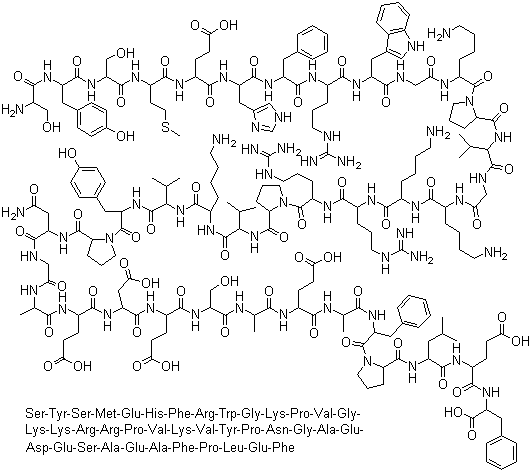
Corticotropin
CAS:9002-60-2
-
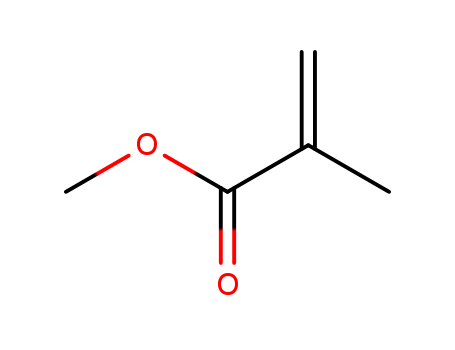
Poly(methyl methacrylate)
CAS:9011-14-7
-
Poly(butylene terephthalate)
CAS:26062-94-2