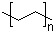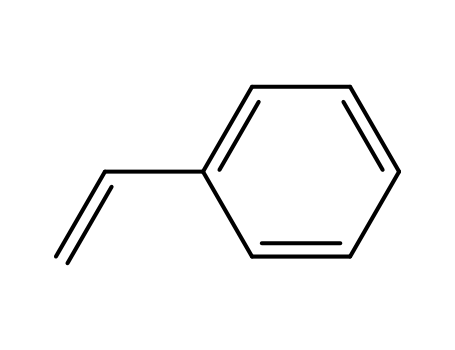
9003-53-6
- Product Name:Polystyrene
- Molecular Formula:(C8H8)n
- Purity:99%
- Molecular Weight:104.152
Product Details;
CasNo: 9003-53-6
Molecular Formula: (C8H8)n
Appearance: white powder or beads, or clear solid
Factory Supply Wholesale Polystyrene 9003-53-6 with Cheapest Price
- Molecular Formula:C8H8
- Molecular Weight:104.152
- Appearance/Colour:white powder or beads, or clear solid
- Melting Point:212 °C
- Refractive Index:n20/D 1.5916
- Boiling Point:212℃
- Flash Point:>230 °F
- PSA:0.00000
- Density:1.06 g/mL at 25 °C
- LogP:2.32960
Poly(styrene)(Cas 9003-53-6) Usage
|
Chemical Properties |
Poly(styrene) is white powder or beads, or clear solid.Polystyrene is rigid with excellent dimensional stability, has good chemical resistance to aqueous solutions, and is an extremely clear material. Impact polystyrene contains polybutadiene added to reduce brittleness. The polybutadiene is usually dispersed as a discrete phase in a continuous polystyrene matrix. Polystyrene can be grafted onto rubber particles, which assures good adhesion between the phases. |
|
Uses |
Containers, tubs, and trays formed from extruded impact polystyrene sheets are used for packaging a large variety of food. Biaxially oriented polystyrene film is thermoformed into blister packs, meat trays, container lids, and cookie, candy, pastry, and other food packages where clarity is required. Housewares is another large segment of the use of polystyrenes. Refrigerator door liners and furniture panels are typical thermoformed impact polystyrene applications. Extruded profiles of solid or foamed impact polystyrene are used for mirror or picture frames, and moldings for construction applications. General-purpose polystyrene is extruded either clear or embossed for room dividers, shower doors, glazings, and lighting applications. Injection molding of impact polystyrene is used for household items, such as flower pots, personal care products, and toys. General-purpose polystyrene is used for cutlery, bottles, combs, disposable tumblers, dishes, and trays. Injection blow molding can be used to convert polystyrene into bottles, jars, and other types of open containers. Impact polystyrene with ignition-resistant additives is used for appliance housings, such as those for television and small appliances. Structural foam impact polystyrene modified with flame-retardant additives is used for business machine housings and in furniture because of its decorability and ease of processing. Consumer electronics, such as cassettes, reels, and housings, is a fast growing area for use of polystyrenes. Medical applications include sample collectors, petri dishes, and test tubes. In an effort to make homes and other buildings more energy efficient, the use of polystyrenes in extruded foam board with flame-retardant additives for walls and under slabs has experienced exceptional growth in recent years. Used as a sheeting material, extruded foam board complies with the requirements of the major building codes as well as federal and military specifications. |
|
Definition |
ChEBI: A polymer composed of repeating ethyl benzene groups. |
|
Preparation |
Styrene may be polymerized by means of all four techniques by bulk, solution, suspension and emulsion polymerization. Each of these methods is practised commercially, but solution polymerization is now the most extensively used. The four processes are described below.(a) Bulk polymerization In a common type of process, styrene is partially polymerized batch-wise by heating the monomer (without added initiator) in large vessels at about 80°C for 2 days until about 35% conversion is attained. The viscous solution of polymer in monomer is then fed continuously into the top of a tower which is some 25 feet high. The top of the tower is maintained at a temperature of about 100°C, the centre at about 150°C and the bottom at about 180°C. As the feed material traverses the temperature gradient, polymerization occurs and fully polymerized material emerges from the base of the tower. The reaction is controlled by a complex array of heating and cooling jackets and coils with which the tower is fitted. The molten material is fed into an extruder, extruded as filament and then cooled and chopped into granules. Since the product contains few impurities, it has high clarity and good electrical insulation properties. The polymer has a broader molecular weight distribution than polymer prepared at one temperature.(b) Solution polymerization Continuous solution processes have found wide commercial utilization, the main advantage over bulk methods being a lessening of the problems associated with the movement and heat transfer of viscous masses. However, the technique does require the added steps of solvent removal and recovery. Typically, a mixture of monomer, solvent (3-12% ethylbenzene) and initiator is fed into a train of three polymerization reactors, each with several heating zones. The reaction temperature is progressively increased, rising from 110-130°C in the first reactor to 150-170°C in the last. The polymer solution is then extruded as fine strands into a devolatilizing vessel. In this vessel, which is at a temperature of 225°C, removal of solvent and unreacted monomer takes place, being aided by the large surface area of the strands. The molten material is fed into an extruder, extruded as filament, cooled and chopped. It may be noted that this type of process is commonly regarded as a continuous bulk process since the amount of solvent used is so small.(c) Suspension polymerization Suspension processes simplify the heat transfer problems associated with bulk methods and, unlike solution methods, they do not involve solvent removal and recovery. The disadvantages of the suspension technique are that it requires the added step of drying and it does not readily lend itself to continuous operation. Typically, polymerization is carried out batch-wise in a stirred reactor, jacketed for heating and cooling.Reaction temperature is about 90°C. When polymerization is complete, the product, in the form of a slurry, is washed with hydrochloric acid and water to remove suspending agent, centrifuged, dried in warm air (at about 60°C), extruded and chopped. (d) Emulsion polymerization Emulsion processes are not used for making solid grades of polystyrene. This is because these processes lead to polymer containing large quantities of soap residues which impair the electrical insulation properties and optical clarity. Emulsion polymerization does, however, find limited application in the production of polystyrene latex used in water-based surface coatings. The techniques employed are very similar to those used for other polymer latices, e.g. poly(vinyl acetate) latex. |
|
General Description |
Polystyrene (for GPC, 4,000) is a synthetic thermoplastic, that is attractive for a wide range of applications because of its properties such as low cost, rigidity, low specific weight, high chemical resistance, mechanical flexibility, biocompatibility and good processability. |
|
Hazard |
Questionable carcinogen. |
|
Industrial uses |
Polystyrene is brittle at room temperature,becomes soft at 80°C, and is often modified bycopolymerization. Traditionally, it is used infilm form for capacitors, and it remains competitivefor this application. Poly styrene is alsoused for coaxial-cable insulation, but in woundstrip or bead form, because the solid is not veryflexible. |
|
Safety Profile |
Questionable carcinogen with experimental tumorigenic data by implant. When heated to decomposition it emits acrid smoke and irritating fumes. See also POLYMERS, IN SOLUBLE. |
|
Purification Methods |
Precipitate polystyrene repeatedly from CHCl3 or toluene solution by addition of MeOH. Dry it in vacuo. [Miyasaka et al. J Phys Chem 92 249 1988.] |
InChI:InChI=1/C9H8/c1-8(2)9-6-4-3-5-7-9/h1-8H
9003-53-6 Relevant articles
Photoredox Catalyzed Sulfonylation of Multisubstituted Allenes with Ru(bpy)3Cl2 or Rhodamine B
Chen, Jingyun,Chen, Shufang,Jiang, Jun,Lu, Qianqian,Shi, Liyang,Xu, Zekun,Yimei, Zhao
supporting information, (2021/11/09)
A highly regio- and stereoselective sulf...
Selective C(sp3)?N Bond Cleavage of N,N-Dialkyl Tertiary Amines with the Loss of a Large Alkyl Group via an SN1 Pathway
Bai, Lu,Li, Linqiang,Liu, Mengtian,Luan, Xinjun,Wu, Jiaoyu
supporting information, (2021/12/01)
Polar disconnection of the C(sp3)?N bond...
Palladium nanoparticlesin situsynthesized onCyclea barbatapectin as a heterogeneous catalyst for Heck coupling in water, the reduction of nitrophenols and alkynes
Le, Van-Dung,Le, T. Cam-Huong,Chau, Van-Trung,Le, T. Ngoc-Duyen,Dang, Chi-Hien,Vo, T. To-Nguyen,Nguyen, Trinh Duy,Nguyen, Thanh-Danh
, p. 4746 - 4755 (2021/03/22)
This study develops an effective method ...
A stable well-defined copper hydride cluster consolidated with hemilabile phosphines
Yuan, Shang-Fu,Luyang, Heng-Wang,Lei, Zhen,Wan, Xian-Kai,Li, Jiao-Jiao,Wang, Quan-Ming
, p. 4315 - 4318 (2021/05/05)
Copper hydrides are very useful in hydro...
9003-53-6 Process route
-
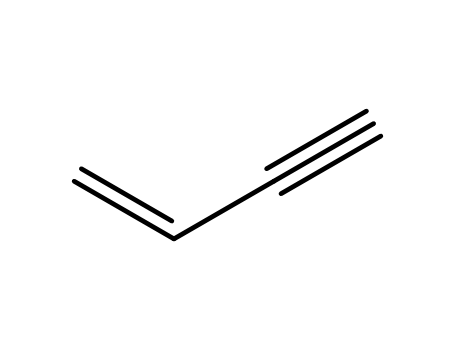
- 689-97-4
3-buten-1-yne

-
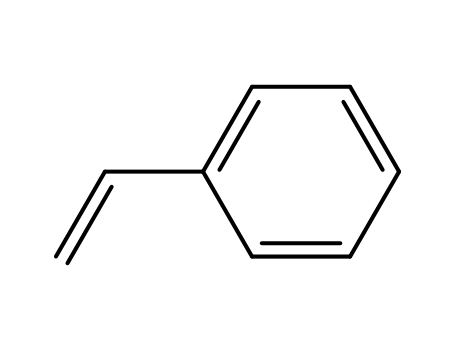
- 100-42-5,25038-60-2,25247-68-1,28213-80-1,28325-75-9,79637-11-9,9003-53-6
styrene
| Conditions | Yield |
|---|---|
|
With acetic acid; at 105 ℃;
|
|
|
With methanol; at 150 ℃;
|
-
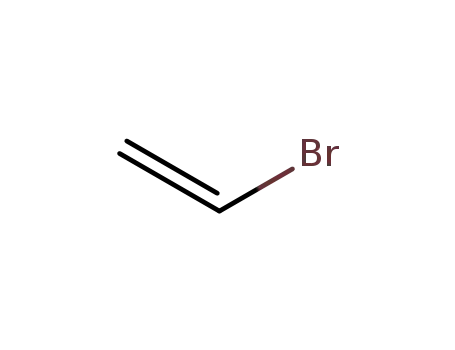
- 593-60-2,25951-54-6
Vinyl bromide

-
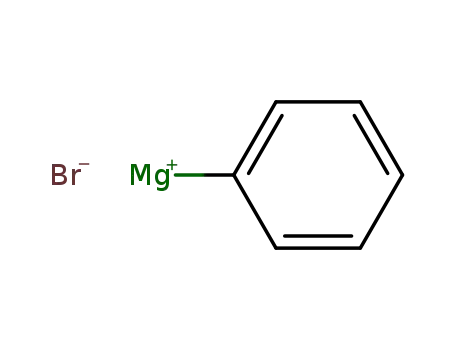
-
phenylmagnesium bromide

-

- 100-42-5,25038-60-2,25247-68-1,28213-80-1,28325-75-9,79637-11-9,9003-53-6
styrene
| Conditions | Yield |
|---|---|
|
With diethyl ether;
|
|
|
With chromium chloride; diethyl ether;
|
|
|
With chromium chloride; diethyl ether;
|
|
|
With diethyl ether;
|
9003-53-6 Upstream products
-
75-21-8
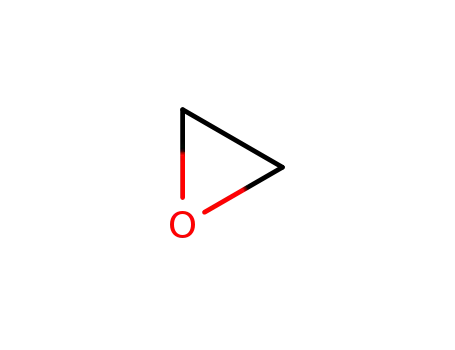
oxirane
-
100-41-4
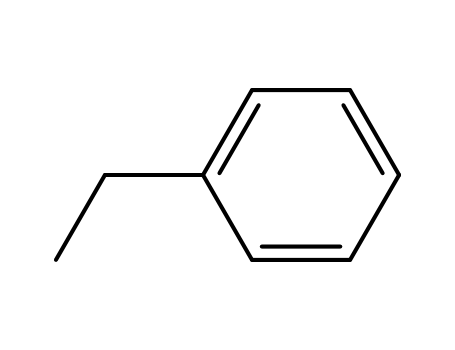
ethylbenzene
-
104-51-8
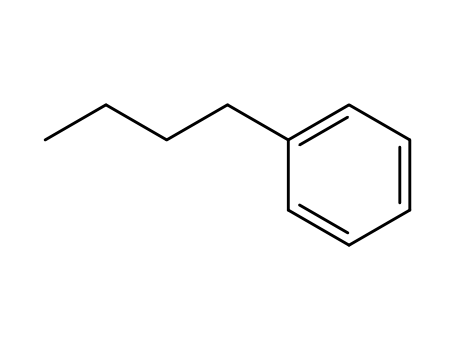
1-butylbenzene
-
110-86-1
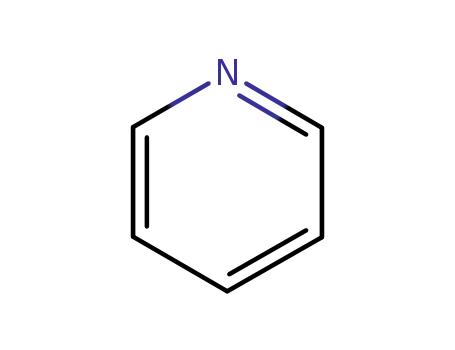
pyridine
9003-53-6 Downstream products
-
39657-33-5
2-methyl-1-phenethyl-aziridine
-
936-48-1
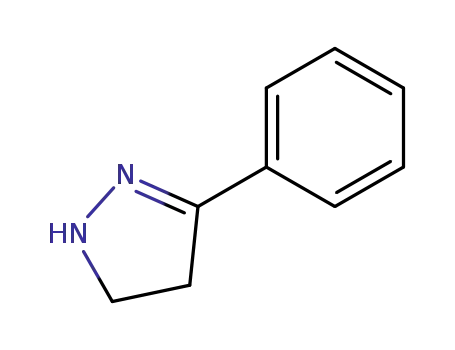
3-phenyl-4,5-dihydro-1H-pyrazole
-
13865-49-1
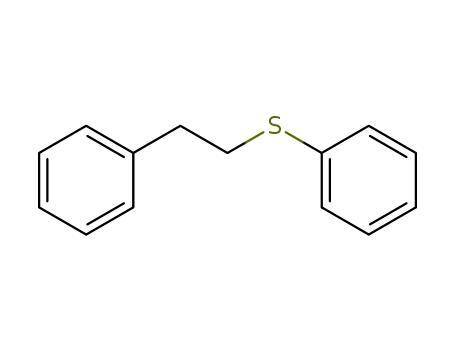
[2-(phenylsulfanyl)ethyl]benzene
-
57308-70-0
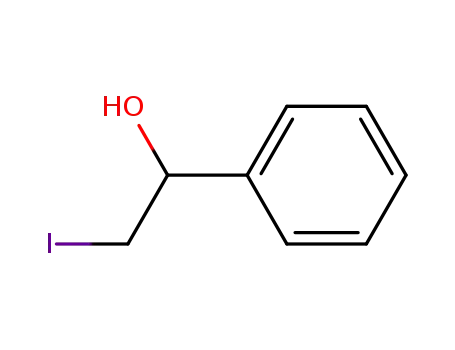
2-iodo-1-phenylethan-1-ol
Relevant Products
-
Polythene
CAS:9002-88-4
-
Polycarbonate resin
CAS:25037-45-0
-
Polylactic acid
CAS:26023-30-3