2835-99-6
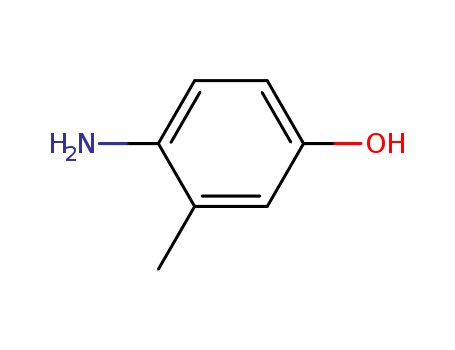
- Product Name:4-Amino-3-methylphenol
- Molecular Formula:C7H9NO
- Purity:99%
- Molecular Weight:123.155
Product Details;
CasNo: 2835-99-6
Molecular Formula: C7H9NO
Appearance: white crystal powder
Chinese Factory Supply Hot Sale 4-Amino-3-methylphenol 2835-99-6 with Safe Shipping
- Molecular Formula:C7H9NO
- Molecular Weight:123.155
- Appearance/Colour:white crystal powder
- Vapor Pressure:1.65mmHg at 25°C
- Melting Point:176-179 °C(lit.)
- Refractive Index:1.424
- Boiling Point:286.7 °C at 760 mmHg
- PKA:10.34±0.18(Predicted)
- Flash Point:127.2 °C
- PSA:46.25000
- Density:1.157 g/cm3
- LogP:1.86400
4-Amino-m-cresol(Cas 2835-99-6) Usage
|
Description |
4-Amino-3-methylphenol is a metabolite of 3-methyl-4-nitrophenol. It is a major metabolite of carcinogenic o-toluidine and causes DNA damage in the presence of Cu(II). |
|
Chemical Properties |
Light gray powder |
|
Uses |
4-Amino-3-methylphenol is a useful reagent for the synthesis of piperidine (piperazine)-amide substituted derivatives as multi-target antipsychotics.4-Amino-3-methylphenol was used in synthesis of a new type of tweezer-molecule in which a strongly preferred binding conformation is generated by convergent, intramolecular hydrogen bonding. |
|
Application |
4-Amino-m-cresol is used as an oxidative hair dye at a maximum concentration of 1.5% after mixing with peroxide. |
|
Definition |
ChEBI: A substituted aniline in which the aniline ring carries 4-hydroxy and 6-methyl substituents; a urinary metabolite of lidocaine. |
|
General Description |
4-Amino-3-methylphenol is a metabolite of 3-methyl-4-nitrophenol. It is a major metabolite of carcinogenic o-toluidine and causes DNA damage in the presence of Cu(II). |
|
Flammability and Explosibility |
Notclassified |
|
Synthesis |
The synthesis of 4-Amino-3-methylphenol is as follows:A mixture of the para-benzoquinone mono-oxime (1.26 mmol),SnCl2 (0.72 g, 3.80 mmol), 20 mL of CH2Cl2, and 0.2 mL of concentrated HCl, was heated to reflux for 16 h. The CH2Cl2 was removed under reduced pressure, and the residue was dissolved in ethyl acetate and washed with concentrated aqueous NaHCO3. The organic layer was dried over anhydrous Na2SO4 and the filtrate was concentrated under reduced pressure to afford the solid product. |
|
Purification Methods |
Crystallise it from 50% EtOH. [Beilstein 13 H 593, 13 IV 1698.] |
InChI:InChI=1/C8H14O3/c1-5-6-10-7(9)11-8(2,3)4/h5H,1,6H2,2-4H3
2835-99-6 Relevant articles
New route for synthesis of fluorescent SnO2 nanoparticles for selective sensing of Fe(III) in aqueous media
Vyas, Gaurav,Kumar, Anshu,Bhatt, Madhuri,Bhatt, Shreya,Paul, Parimal
, p. 3954 - 3959 (2018)
A simple new route for synthesis of fluo...
Hydroxylation of aromatic amines with dioxygen in photooxidation sensitized by substituted phthalocyanines
Fedorova, Tatyana M.,Derkacheva, Valentina M.,Shevchenko, Ekaterina N.,Luk'yanets, Evgeny A.,Bordaev, Eduard B.,Kaliya, Oleg L.
, p. 64 - 66 (2020/03/03)
Photooxidation of aniline and its methyl...
A capping agent dissolution method for the synthesis of metal nanosponges and their catalytic activity towards nitroarene reduction under mild conditions
Ghosh, Sourav,Jagirdar, Balaji R.
, p. 17401 - 17411 (2019/01/03)
We report a general strategy for the syn...
Monodispersed Ag nanoparticles as catalyst: Preparation based on crystalline supramolecular hybrid of decamethylcucurbit[5]uril and silver ions
Li, Hong-Fang,Lue, Jian,Lin, Jing-Xiang,Cao, Rong
, p. 5692 - 5697 (2014/06/23)
Monodispersed silver nanoparticles (Ag0 ...
2835-99-6 Process route
-
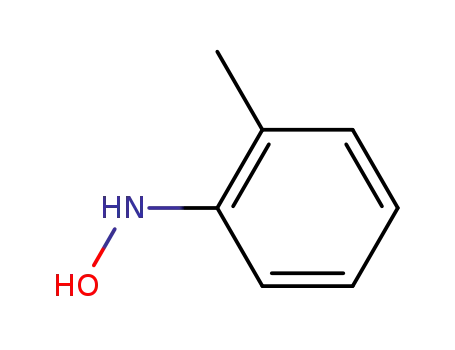
- 611-22-3
N-(o-tolyl)hydroxylamine

-
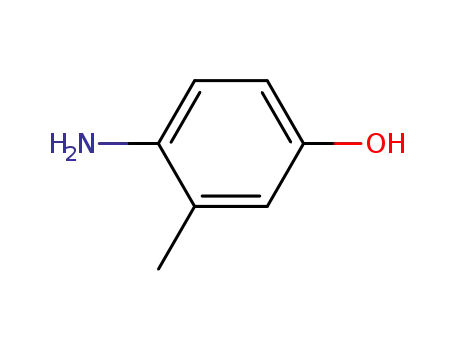
- 2835-99-6
4-amino-3-methylphenol

-
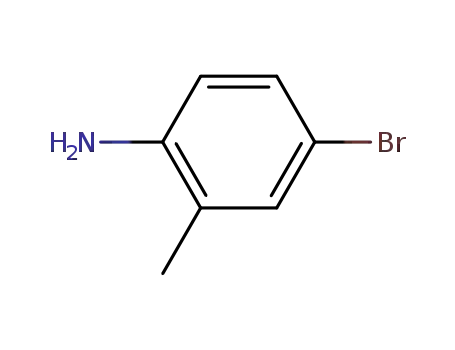
- 583-75-5
4-Bromo-2-methylaniline

-
- 95-53-4
o-toluidine
| Conditions | Yield |
|---|---|
|
With perchloric acid; sodium perchlorate; sodium bromide; In water; acetonitrile; at 25 ℃; Rate constant;
|
-
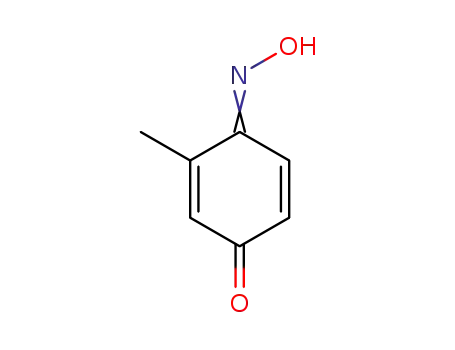
- 13362-34-0
3-methyl-1,4-benzoquinone 4-oxime

-

- 2835-99-6
4-amino-3-methylphenol
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; tin(ll) chloride; In dichloromethane; water; for 16h; Reagent/catalyst; Reflux;
|
88% |
2835-99-6 Upstream products
-
2581-34-2
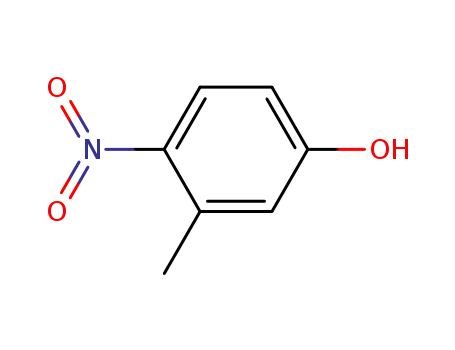
3-methyl-4-nitrophenol
-
88-72-2
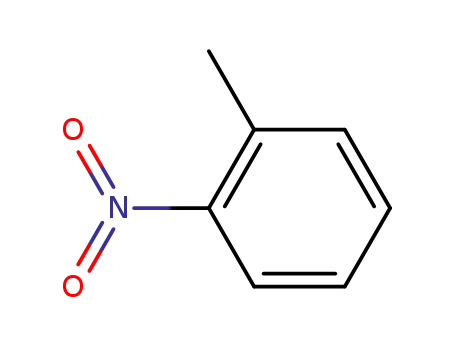
1-methyl-2-nitrobenzene
-
2724-87-0
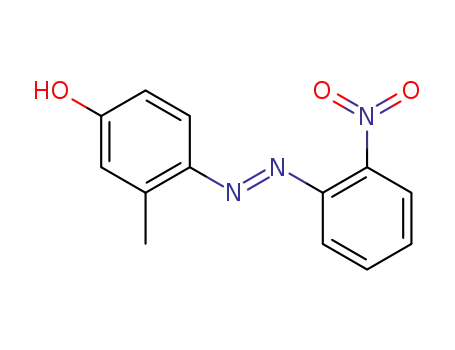
3-Methyl-4-(2-nitro-phenylazo)-phenol
-
108-39-4
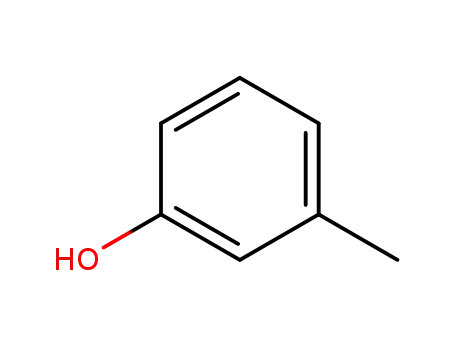
3-methyl-phenol
2835-99-6 Downstream products
-
100868-09-5
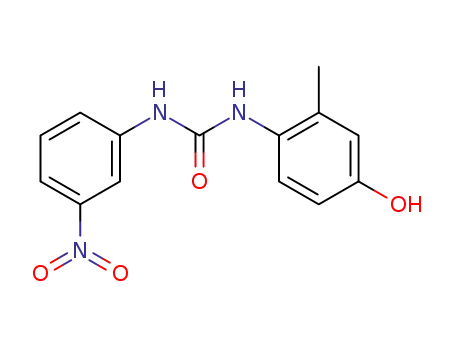
N-(4-hydroxy-2-methyl-phenyl)-N'-(3-nitro-phenyl)-urea
-
4370-76-7
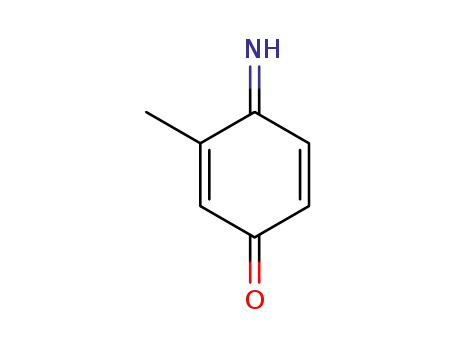
methyl-[1,4]benzoquinone-1-imine
-
133921-27-4
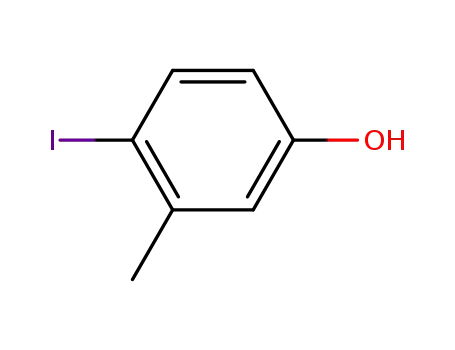
4-iodo-3-methyl-phenol
-
108981-59-5
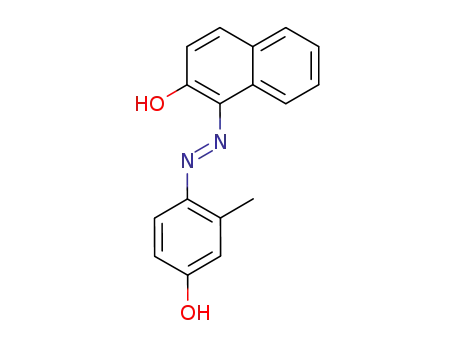
1-(4-Hydroxy-2-methyl-phenylazo)-[2]naphthol
Relevant Products
-
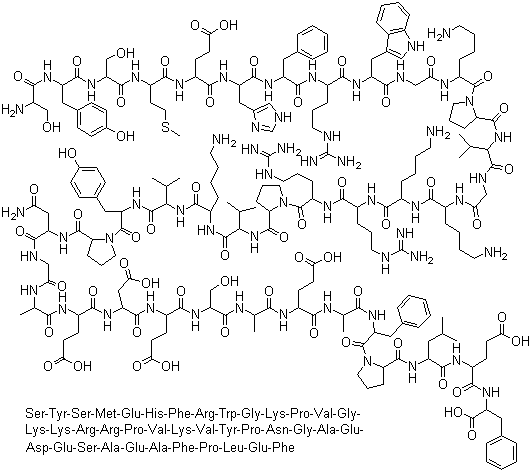
Corticotropin
CAS:9002-60-2
-
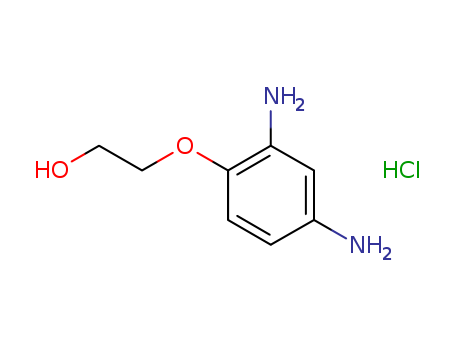
2-(2,4-Diaminophenoxy)ethanol dihydrochloride
CAS:66422-95-5
-
Tea tree oil
CAS:68647-73-4