9004-57-3
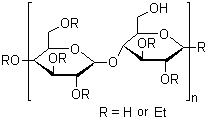
- Product Name:Ethyl cellulose
- Molecular Formula:Unspecified
- Purity:99%
- Molecular Weight:448.47400
Product Details;
CasNo: 9004-57-3
Molecular Formula: Unspecified
Appearance: white powder
Trustworthy Factory Supply Buy High Grade Ethyl cellulose 9004-57-3 with Competitive Price
- Molecular Formula:Unspecified
- Molecular Weight:448.47400
- Appearance/Colour:white powder
- Melting Point:240-255oC
- Refractive Index:n20/D 1.47(lit.)
- PSA:0.00000
- Density:1.45
- LogP:0.00000
Ethyl cellulose(Cas 9004-57-3) Usage
|
Chemical Properties |
Ethylcellulose is a tasteless, free-flowing, white to light tan-colored powder. |
|
Uses |
ethyl cellulose is a binder, film former, and thickener. It is used in suntan gels, creams, and lotions. This is the ethyl ether of cellulose. |
|
Production Methods |
Ethylcellulose is prepared by treating purified cellulose (sourced from chemical-grade cotton linters and wood pulp) with an alkaline solution, followed by ethylation of the alkali cellulose with chloroethane as shown below, where R represents the cellulose radical: RONa + C2H5Cl→ROC2H5+NaCl The manner in which the ethyl group is added to cellulose can be described by the degree of substitution (DS). The DS designates the average number of hydroxyl positions on the anhydroglucose unit that have been reacted with ethyl chloride. Since each anhydroglucose unit of the cellulose molecule has three hydroxyl groups, the maximum value for DS is three. |
|
Preparation |
Ethyl cellulose is prepared by reacting cellulose with caustic to form caustic cellulose, which is then reacted with chloroethane to form ethyl cellulose. Plasticgrade material contains 44-48% ethoxyl. Although not as resistant as cellulose esters to acids, it is much more resistant to bases. An outstanding feature is its toughness at low temperatures. |
|
Brand name |
Aquacoat ECD (FMC);Ethocel (Dow Chemical). |
|
Pharmaceutical Applications |
Ethylcellulose is widely used in oral and topical pharmaceutical formulations. The main use of ethylcellulose in oral formulations is as a hydrophobic coating agent for tablets and granules.Ethylcellulose coatings are used to modify the release of a drug, to mask an unpleasant taste, or to improve the stability of a formulation; for example, where granules are coated with ethylcellulose to inhibit oxidation. Modified-release tablet formulations may also be produced using ethylcellulose as a matrix former. Ethylcellulose, dissolved in an organic solvent or solvent mixture, can be used on its own to produce water-insoluble films. Higher-viscosity ethylcellulose grades tend to produce stronger and more durable films. Ethylcellulose films may be modified to alter their solubility, by the addition of hypromellose or a plasticizer. An aqueous polymer dispersion (or latex) of ethylcellulose such as Aquacoat ECD (FMC Biopolymer) or Surelease (Colorcon) may also be used to produce ethylcellulose films without the need for organic solvents. Drug release through ethylcellulose-coated dosage forms can be controlled by diffusion through the film coating. This can be a slow process unless a large surface area (e.g. pellets or granules compared with tablets) is utilized. In those instances, aqueous ethylcellulose dispersions are generally used to coat granules or pellets. Ethylcellulose-coated beads and granules have also demonstrated the ability to absorb pressure and hence protect the coating from fracture during compression. High-viscosity grades of ethylcellulose are used in drug microencapsulation. Release of a drug from an ethylcellulose microcapsule is a function of the microcapsule wall thickness and surface area. In tablet formulations, ethylcellulose may additionally be employed as a binder, the ethylcellulose being blended dry or wetgranulated with a solvent such as ethanol (95%). Ethylcellulose produces hard tablets with low friability, although they may demonstrate poor dissolution. Ethylcellulose has also been used as an agent for delivering therapeutic agents from oral (e.g. dental) appliances. In topical formulations, ethylcellulose is used as a thickening agent in creams, lotions, or gels, provided an appropriate solvent is used. Ethylcellulose has been studied as a stabilizer for emulsions. Ethylcellulose is additionally used in cosmetics and food products. |
|
Safety |
Ethylcellulose is widely used in oral and topical pharmaceutical formulations. It is also used in food products. Ethylcellulose is not metabolized following oral consumption and is therefore a noncalorific substance. Because ethylcellulose is not metabolized it is not recommended for parenteral products; parenteral use may be harmful to the kidneys. Ethylcellulose is generally regarded as a nontoxic, nonallergenic, and nonirritating material. As ethylcellulose is not considered to be a health hazard, the WHO has not specified an acceptable daily intake. The highest reported level used in an oral product is 308.8 mg in an oral sustained release tablet.LD50 (rabbit, skin): >5 g/kg LD50 (rat, oral): >5 g/kg |
|
storage |
Ethylcellulose is a stable, slightly hygroscopic material. It is chemically resistant to alkalis, both dilute and concentrated, and to salt solutions, although it is more sensitive to acidic materials than are cellulose esters. Ethylcellulose is subject to oxidative degradation in the presence of sunlight or UV light at elevated temperatures. This may be prevented by the use of antioxidant and chemical additives that absorb light in the 230–340nm range. Ethylcellulose should be stored at a temperature not exceeding 32°C (90°F) in a dry area away from all sources of heat. It should not be stored next to peroxides or other oxidizing agents. |
|
Incompatibilities |
Incompatible with paraffin wax and microcrystalline wax. |
|
Regulatory Status |
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral capsules, suspensions and tablets; topical emulsions and vaginal preparations). Included in nonparenteral medicines licensed in Europe. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
|
Who Evaluation |
Evaluation year: 1960 |
Relevant Products
-
Humic acid sodium salt
CAS:68131-04-4
-
Sodium dodecylbenzenesulphonate
CAS:25155-30-0
-
Vancomycin
CAS:1404-90-6