1404-90-6
- Product Name:Vancomycin
- Molecular Formula:C66H75Cl2N9O24
- Purity:99%
- Molecular Weight:1449.27
Product Details;
CasNo: 1404-90-6
Molecular Formula: C66H75Cl2N9O24
Appearance: Almost white powder
Factory Supply High Purity 99% Vancomycin 1404-90-6 In Stock
- Molecular Formula:C66H75Cl2N9O24
- Molecular Weight:1449.27
- Appearance/Colour:Almost white powder
- Refractive Index:1.7350 (estimate)
- PKA:pKa 2.18±0.08(H2O t = 25.0±0.1 I = 0.2 (NaCl) N2 atmosphere) (Uncertain);7.75±0.02(H2O t = 25.0±0.1 I = 0.2 (NaCl) N2 atmosphere) (Uncertain);8.89±0.01(H2O t = 25.0±0.1 I = 0.2 (NaCl) N2 atmosphere) (Uncertain)
- PSA:530.49000
- Density:1.657 g/cm3
- LogP:4.73460
Vancomycin(Cas 1404-90-6) Usage
|
Overview |
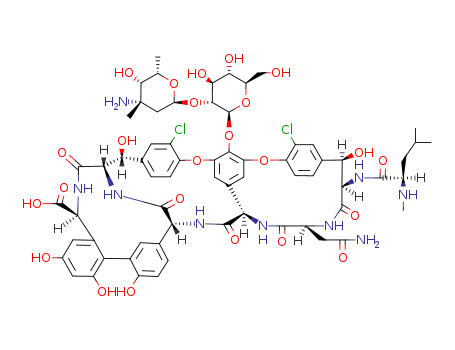
Vancomycin is a complex tricyclic glycopeptide antibiotic produced by Streptococcus orientalis[1, 2]. Vancomycin was first discovered from a soil sample in the interior jungle of Borneo in the 1950s, and its usage was very limited due to the presence of impurities that caused toxicities in the earlier preparations. However, the use of vancomycin was reconsidered after the emergence of methicillin-resistant Staphylococci in the 1970s, and its usage increased from the 1980s after purer preparations were made in late 1970s[3]. Now, vancomycin has become the most common injectable drug of choice to treat methicillin-resistant Staphylococci species and drug resistant Enterococcus species[4]. Figure 1 The chemical structure of vancomycin VCM is generally prescribed to combat severe infections caused by Gram-positive bacteria, to fight microorganisms that are resistant to other antimicrobial agents or still indicated to patients who are allergic to penicillins and cephalosporins[1, 5]. As a consequence, it is very much used in intensive care units (ICU) for the treatment of not only hospital infections and sepsis, but also of pneumonia cases, empyema, endocarditis, osteomyelitis, soft tissue abscesses among others[1, 6, 7]. This drug is not the first-choice agent owing to its adverse effects like hypotension and tachycardia, phlebitis, nephrotoxicity, ototoxicity[7], hypersensibility reactions, chills, exanthema and fever1, and the fact that peripheral IV complications are a major concern[8]. The literature reveals that the use of inappropriate doses and prolonged therapyes increase the risk of toxicity that, in turn, favors the onset of adverse effects[9-11]. Therefore, the people who use antibiotics in animals and humans must be vigilant about the adverse. Therefore, the people who use antibiotics in animals and humans must be vigilant about the adverse effects and the proper doses—especially in case of last-line antibiotics such as vancomycin. 3 doses; however, higher doses may be prescribed1. Although the established pediatric doses vary according to the age range described below[1, 3], no consensus has been reached regarding the adequate dose of vancomycin for children. |
|
Indication and Administration |
Vancomycin is active against most strains of Clostridia, resistant strains), coagulase-negative Staphylococci, and Viridans group Staphylococci and Enterococci. Vancomycin is not effective against Gram-negative bacteria[12]. Vancomycin is one of the antibiotics of last resort, used only after treatment with other antibiotics has failed in the treatment of life-threatening infections by Gram-positive bacteria. Even though vancomycin has great potential in treating infections in animals, the use of vancomycin in veterinary medicine is limited because it is expensive and requires continuous intravenous infusion[13]. Powdered vancomycin is reconstituted in sterile water, which results in a dark-colored solution, and it is further diluted in 5% dextrose or saline when it is administered. The reconstituted solution is stable for 14 days either at room temperature or in a refrigerator. Additionally, 125 mg and 250 mg vancomycin tablets are available for oral administration[14]. |
|
Pharmacodynamic |
The factors that affect the activity of vancomycin are its tissue distribution, its protein-binding, inoculum size, and resistant organisms. The volume of distribution in humans is 0.4–1 L/kg; in dogs, 0.4–5.5 L/kg[15]. The binding of vancomycin to protein has a range from 10% to 50%. A 1–8-fold increase in the minimum inhibitory concentration (MIC) has been shown in several in vitro assessments as a result of the presence of albumin, whereas the presence of serum has had a more variable effect[16]. It is evident in an in vitro pharmacodynamic model that the time taken to kill is longer when the inoculum size is high (9.5 log10 CFU/g) compared to a moderate inoculum (5.5 log10 CFU/g): 48 versus 72 h, respectively, for both the methicillin sensitive Staphylococci and methicillin-resistant Staphylococci organisms isolated from human patients[16,17]. Vancomycin penetrates into most body spaces, and the penetrability is dependent on the degree of inflammation present. The concentration of vancomycin in different body spaces is different[18]. The inflamed meninges improve the penetration of vancomycin into the cerebral spinal fluid, with reported concentrations of 6.4–11.1 mg/L, whereas uninflamed meninges have resulted in low concentrations of 0–3.45 mg/L in humans[19]. Furthermore, it has been shown in a rabbit model that a high concentration of vancomycin is present in the cerebral spinal fluid of inflamed meninges[20]. Therapeutic concentrations of vancomycin in ascitic, pericardial, pleural, and synovial fluids are greater than 2.5 mg/L in humans[21]. More than 80% and 50% of a vancomycin dose is excreted unchanged in the urine (mostly by way of glomerular filtration) within 24 h after administration in humans and dogs, respectively, and the concentration of vancomycin in liver tissue and bile is below detectable levels. Vancomycin has a distribution phase of 30 min to 1 h. The half-life of vancomycin in patients with normal creatinine clearance in humans is about 6 h; dogs, 2 h; horses, 3 h[22]. |
|
Brand Name(s) in US |
Vancocin, Vancoled |
|
Mode of action |
The mechanism of action of this antimicrobial agent is via the inhibition of bacterial cell wall biosynthesis or, more specifically, the inhibition of peptidoglycan biosynthesis. It is, therefore, bactericidal for reproductive bacteria[1]. The bacterial cell wall contains peptidoglycan that encircles the whole bacteria. In Gram-positive bacteria this substance is more significantly present, and it forms large and insoluble layers on the outer part of the cell membrane, totaling up to 40 layers which consist of multiple skeletons of amino sugars: N-acetylglucosamine and N-acetylmuramic[24]. The latter contains lateral short peptide residues with cross-links, which form a high-level resistant polymeric chain. The drug inhibits this polymerization or the transglycosylase reaction once it binds with high affinity to the C-terminal D-alanyl D-alanine residues of lipid-linked cell wall precursors and blocks the linkage to the glycopeptide polymer1. As a result, it hinders the cross-links of peptides from binding to tetrapeptide side chains; namely, it prevents its linkage to the growing tip of the peptidoglycan[24]. |
|
Resistance issue |
Antibiotic use either as therapy, in the prevention of bacterial diseases, or as performance enhancers has resulted in antibiotic-resistant microorganisms in pathogens and among bacteria of the endogenous microflora of animals. Antibiotic-resistant bacteria present in animals can be transmitted to humans via contact or via the food chain. Furthermore, resistance genes of animal bacteria can be transferred to human pathogens in the intestinal flora of humans. The development of intermediate and high levels of resistance to vancomycin for Staphylococcus aureus was reported for the first time in Japan in 1997[25]. According to guidelines of the Clinical Laboratory Standards Institute, susceptibility break points of vancomycin are <4 mcg/mL for Enterococcus, <1 mcg/mL for Streptococcus, and <4 mcg/mL for Staphylococcus. However, in 2006, the vancomycin MIC breakpoints for S. aureus were lowered to 2 ug/mL for “susceptible”, 4–8 ug/mL for “intermediate”, and 16 ug/mL for “resistant”[26]. Enterococci should be regularly tested in vitro for susceptibility to vancomycin for determination of MIC. Enterococci are deemed susceptible to vancomycin if MICs are <4 ug/mL; they are considered as intermediate level resistance to vancomycin if MICs are 8 to 16 ug/mL; and as complete resistance to vancomycin if MICs are >16 ug/mL. |
|
Side effects |
Prolonged intravenous use of vancomycin may cause neutropenia, thrombophlebitis, rash, fever, anemia, thrombocytopenia, and ototoxic reactions in humans and animals. Vancomycin should be administered intravenously in diluted form, because it is highly irritable for the tissues. It may cause local phlebitis at the site of injection in animals[14]. Vancomycin should be infused for over 1h to reduce the risk of the histamine release-associated “red man” syndrome in humans. It is advised not to administer intravenous rapidly, so as to avoid acute adverse reaction in animals[4]. The major drawback of vancomycin usage is auditory damage in humans; however, tinnitus and deafness might improve once the treatment is ceased. In addition to that, nausea, chills, phlebitis, severe hypotension, wheezing, dyspnoea, urticaria, and pruritus have been observed with the treatment of vancomycin in humans[27–30]. In some instances, neutropenia was detected with prolonged therapy[31]. There is a potential for nephrotoxicity and ototoxicity with vancomycin in animals[32]. Toxicities are minimal in vancomycin monotherapy at conventional dosages of 1 g (15 mg/kg) every 12 h in humans[33]. However, increased incidence of nephrotoxicity has been established with doses of 4 g/day or higher. As a result of elevated dosage, serum concentrations may increase, which may lead to toxicity[34]. Vancomycin increases the risk of nephrotoxicity in humans with drugs such as amphotericin, capreomycin, cyclosporine, cisplatin, colistimethate, polymyxins, and tacrolimus. |
|
Description |
Vancomycin is produced by fermentation of Amycol atopsis orientalis (formerly Nocardi a orientalis). It has been available for approximately 40 years, but its popularity has increased significantly with the emergence of MRSA in the early 1980s. Chemically, vancomycin has a glycosy lated hexapeptide chain that is rich in unusual amino acids, many of which contain aromatic rings cross-linked by aryl ether bonds into a rigid molecular framework. |
|
Originator |
Vancocin,Lilly,US,1958 |
|
Uses |
Antibacterial. |
|
Definition |
ChEBI: A complex glycopeptide from Streptomyces orientalis. It inhibits a specific step in the synthesis of the peptidoglycan layer in the Gram-positive bacteria Staphylococcus aureus and Clostridium difficile. |
|
Manufacturing Process |
An agar slant is prepared containing the following ingredients: 20 grams starch, 1 gram asparagine, 3 grams beef extract, 20 grams agar, and 1 liter water. The slant is inoculated with spores of S. orientalis, Strain M43-05865, and is incubated for about 10 days at 30°C. The medium is then covered with sterile distilled water and scraped to loosen the spores. The resulting suspension of spores is preserved for further use in the process. A liquid nutrient culture medium is prepared containing the following ingredients: 15 grams glucose, 15 grams soybean meal, 5 grams corn steep solids, 2 grams sodium chloride, 2 grams calcium carbonate, and 1 liter water. The medium is sterilized at 120°C for about 30 minutes in a suitable flask and cooled. 10 ml of a spore suspension prepared as set forth above are used to inoculate the medium. The inoculated medium is shaken for 48 hours at 26°C on a reciprocating shaker having a 2-inch stroke, at 110 rpm. The fermented culture medium which comprises a vegetative inoculum is used to inoculate a nutrient culture medium containing the following ingredients: 20 grams blackstrap molasses, 5 grams soybean peptone, 10 grams glucose, 20 grams sucrose, 2.5 grams calcium carbonate, and 1 liter water. The medium is placed in a container having a suitable excess capacity in order to insure the presence of sufficient oxygen and is sterilized by heating at 120°C for about 30 minutes. When cool, the medium is inoculated with about 25 ml of a vegetative inoculum as described above, and the culture is then shaken for about 80 hours at 26°C. The pH of the medium at the beginning of fermentation ranges from about 6.5 to about 7.0 and the final pH is about 7.0 to about 8.0. A fermentation broth thus obtained contained about 180 μg of vancomycin per ml. |
|
Brand name |
Vancocin Hydrochloride (ViroPharma); Vancoled (Baxter Healthcare); Vancor (Pharmacia & Upjohn). |
|
Therapeutic Function |
Antibacterial |
|
Acquired resistance |
Only very recently, despite decades of intensive use, have some vancomycin-resistant bacteria emerged (vancomycin-resistant enterococcus [VRE] and vancomycin-resistant Staphylococcus aureus) [VRSA]. It is alleged that these resistant strains emerged as a consequence of the agricultural use of avoparcin, a structurally related antibiotic that has not found use for human infections in the United States but was used in Europe before its recent ban. The mechanism of resistance appears to be alteration of the target D-alanyl-D-alanine units on the peptidoglycan cell wall precursors to D-alanyl-D-lactate. This results in lowered affinity for vancomycin due to lack of a key hydrogen bonding interaction. It is greatly feared that this form of resistance will become common in the bacteria for which vancomycin is presently the last sure hope for successful chemotherapy. If so, such infection would become untreatable. These resistant strains are not yet common in clinically relevant strains, but most authorities believe that this is only a question of time. Vancomycin-intermediate S.aureus, also called glycoprotein-intermediate S.aureus (VISA), also has been reported. It appears to be resistant because of a thickened peptidoglycan layer. |
|
Mechanism of action |
Vancomycin is a bacterial cell wall biosynthesis inhibitor. Evidence suggests that the active species is a homodimer of two vancomycin units. The binding site for its target is a peptide-lined cleft having high affinity for acetyl-D-alanyl-D-alanine and related peptides through five hydrogen bonds. It inhibits both transglycosylases (inhibiting the linking between muramic acid and acetyl glucosamine units) and transpeptidase (inhibiting peptide cross-linking) activities in cell wall biosynthesis. Thus, vancomycin functions like a peptide receptor and interrupts bacterial cell wall biosynthesis at the same step as the β-lactams do, but by a different mechanism. By covering the substrate for cell wall transamidase, it prevents cross-linking resulting in osmotically defective cell walls. |
|
Clinical Use |
Although a number of adverse effects can result from IV infusion, vancomycin has negligible oral activity. It can be used orally for action in the GI tract, especially in cases of Cl ostri di um difficile overgrowth. The useful spectrum is restricted to Gram-positive pathogens, with particular utility against multiply-resistant, coagulase-negative staphylococci and MRSA, which causes septicemias, endocarditis, skin and soft-tissue infections, and infections associated with venous catheters. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antibacterials: increased risk of nephrotoxicity and ototoxicity with aminoglycosides, capreomycin or colismethate sodium; increased risk of nephrotoxicity with polymyxins. Ciclosporin: variable response; increased risk of nephrotoxicity. Diuretics: increased risk of ototoxicity with loop diuretics. Muscle relaxants: enhanced effects of suxamethonium. Tacrolimus: possible increased risk of nephrotoxicity |
|
Metabolism |
Little or no metabolism of vancomycin is thought to take place. It is excreted unchanged by the kidneys, mostly by glomerular filtration. There is a small amount of nonrenal clearance, although the mechanism for this has not been determined. |
InChI:InChI=1/C66H75Cl2N9O24/c1-23(2)12-34(71-5)58(88)76-49-51(83)26-7-10-38(32(67)14-26)97-40-16-28-17-41(55(40)101-65-56(54(86)53(85)42(22-78)99-65)100-44-21-66(4,70)57(87)24(3)96-44)98-39-11-8-27(15-33(39)68)52(84)50-63(93)75-48(64(94)95)31-18-29(79)19-37(81)45(31)30-13-25(6-9-36(30)80)46(60(90)77-50)74-61(91)47(28)73-59(89)35(20-43(69)82)72-62(49)92/h6-11,13-19,23-24,34-35,42,44,46-54,56-57,65,71,78-81,83-87H,12,20-22,70H2,1-5H3,(H2,69,82)(H,72,92)(H,73,89)(H,74,91)(H,75,93)(H,76,88)(H,77,90)(H,94,95)/t24-,34+,35-,42+,44-,46+,47+,48-,49+,50-,51+,52+,53+,54-,56+,57+,65-,66-/m0/s1
1404-90-6 Relevant articles
Expression and assay of an N-methyltransferase involved in the biosynthesis of a vancomycin group antibiotic
O'Brien, Dominic P.,Kirkpatrick, Peter N.,O'Brien, Simon W.,Staroske, Thomas,Richardson, Timothy I.,Evans, David A.,Hopkinson, Andrew,Spencer, Jonathan B.,Williams, Dudley H.
, p. 103 - 104 (2000)
An N-methyltransferase responsible for m...
Enzymatic glycosylation of vancomycin aglycon: Completion of a total synthesis of vancomycin and N- and C-terminus substituent effects of the aglycon substrate
Nakayama, Atsushi,Okano, Akinori,Feng, Yiqing,Collins, James C.,Collins, Karen C.,Walsh, Christopher T.,Boger, Dale L.
supporting information, p. 3572 - 3575 (2014/07/21)
Studies on the further development of th...
A systematic investigation of the synthetic utility of glycopeptide glycosyltransferases
Oberthuer, Markus,Leimkuhler, Catherine,Kruger, Ryan G.,Lu, Wei,Walsh, Christopher T.,Kahne, Daniel
, p. 10747 - 10752 (2007/10/03)
Glycosyltransferases involved in the bio...
Solid- and solution-phase synthesis of vancomycin and vancomycin analogues with activity against vancomycin-resistant bacteria
Nicolaou,Cho, Suk Young,Hughes, Robert,Winssinger, Nicolas,Smethurst, Christian,Labischinski, Harald,Endermann, Rainer
, p. 3798 - 3823 (2007/10/03)
Vancomycin, the prototypical member of t...
New selenium-based safety-catch linkers: Solid-phase semisynthesis of vancomycin
Nicolaou,Winssinger, Nicolas,Hughes, Robert,Smethurst, Christopher,Cho, Suk Young
, p. 1084 - 1088 (2007/10/03)
Pro-allyl and pro-alloc linkers can be f...
1404-90-6 Process route
-
-
C82H87Cl2N9O28

-
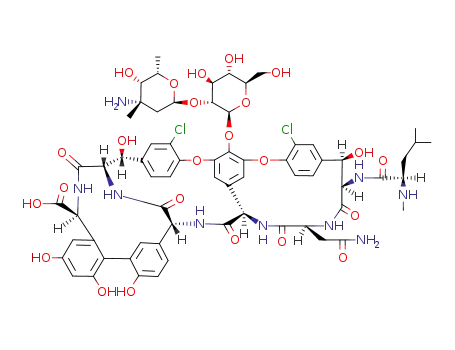
- 1404-90-6
vancomycin
| Conditions | Yield |
|---|---|
|
With ammonium bicarbonate; palladium on activated charcoal; In acetic acid; at 23 ℃; for 4h;
|
81% |
|
With ammonium formate; acetic acid; 10percent Pd/C; In methanol; at 23 ℃; for 4h;
|
81% |
-
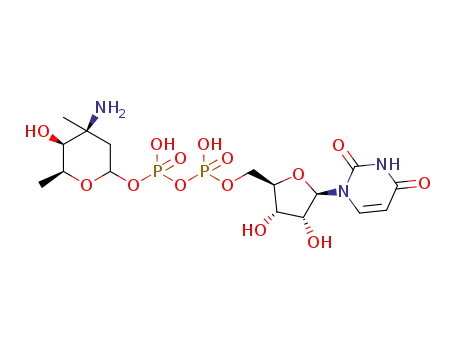
-
UDP-vancosamine

-
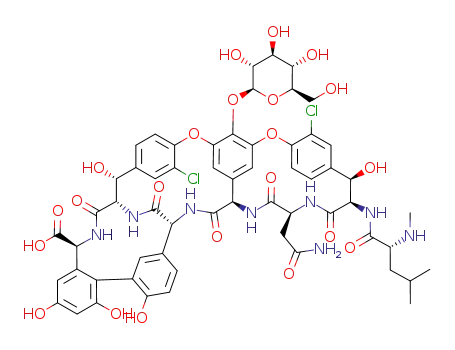
- 101485-50-1
desvancosaminyl vancomycin

-

- 1404-90-6
vancomycin
| Conditions | Yield |
|---|---|
|
With tris(2-carboxyethyl)phosphine; glycosyltransferase GtfD; bovine serum albumin; In glycerol; at 37 ℃; for 1.5h; Enzymatic reaction;
|
87% |
1404-90-6 Upstream products
-
216668-88-1
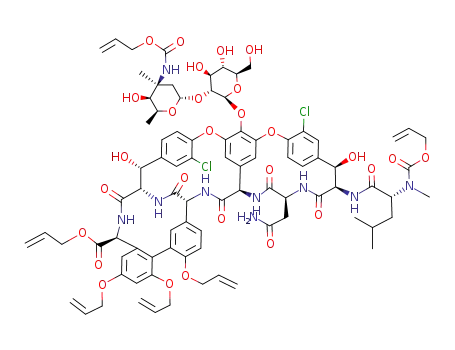
N,N'-dialloc-tri-O-allyl vancomycin allyl ester
-
101485-50-1

desvancosaminyl vancomycin
-
755033-86-4
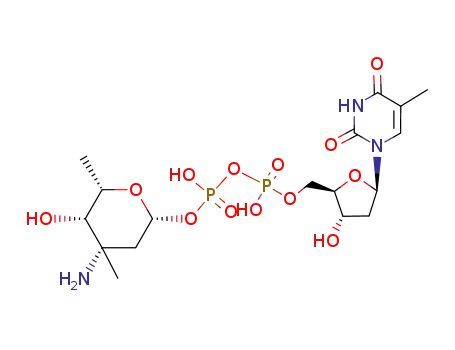
thymidine 5'-(3-amino-2,3,6-trideoxy-3C-methyl-β-L-lyxo-hexopyranosyl diphosphate)
1404-90-6 Downstream products
-
82198-76-3
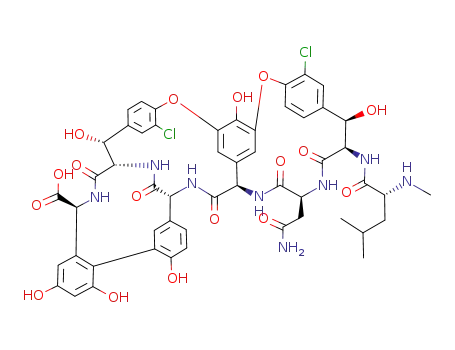
vancomycin aglycon
-
101485-50-1

desvancosaminyl vancomycin
Relevant Products
-
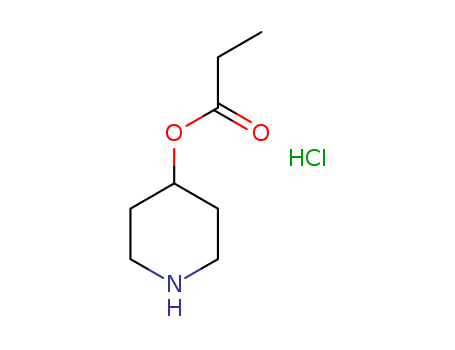
4-PIPERIDINOL, PROPIONATE, HYDROCHLORIDE
CAS:219859-83-3
-
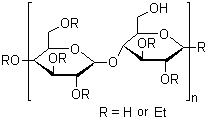
Ethyl cellulose
CAS:9004-57-3
-
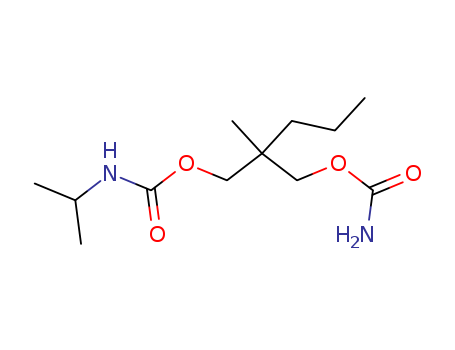
Carisoprodol
CAS:78-44-4