50-67-9
- Product Name:5-Hydroxytryptamine(5-HT)
- Molecular Formula:C10H12N2O
- Purity:99%
- Molecular Weight:176.218
Product Details;
CasNo: 50-67-9
Molecular Formula: C10H12N2O
Appearance: White powder
Trustworthy Factory Supply 99% Pure 5-Hydroxytryptamine(5-HT) 50-67-9 In Stock
- Molecular Formula:C10H12N2O
- Molecular Weight:176.218
- Appearance/Colour:White powder
- Vapor Pressure:1.63E-07mmHg at 25°C
- Melting Point:22-23°C(lit.)
- Refractive Index:1.71
- Boiling Point:416.089 °C at 760 mmHg
- PKA:9.8(at 25℃)
- Flash Point:205.443 °C
- PSA:62.04000
- Density:1.288 g/cm3
- LogP:2.07500
5-Hydroxytryptamine(Cas 50-67-9) Usage
|
Description |
Serotonin is the baby boomer of neurotransmitters: It was identified in the late 1940s, its adolescence was troubled and turbulent, it made the drug scene in the 1960s, and it nearly died of an overdose in the early 1970s. At one point, the remark was made that serotonin doesn't do anything. On reaching its middle years, serotonin has matured and become an important topic of study, a household name, and more complicated than ever. Serotonin has been associated with, among other things, anxiety, depression, schizophrenia, drug abuse, sleep, dreaming, hallucinogenic activity, headache, cardiovascular disorders, and appetite control, and it is now dabbling in acupuncture and transcendental meditation. Serotonin was independently identified in the late 1940s by two groups of investigators: In the United States, it was called serotonin, whereas in Italy, it was called enteramine. Its total synthesis in the early 1950s confirmed that both substances were 5-hydroxytryptamine (5-HT ). Serotonin (5-HT ) was detected in numerous plant and animal species and, in the mid-1950s, was identified in the central nervous system (CNS) of animals. A neurotransmitter role was subsequently proposed for this substance. Later, 5-HT was implicated in a variety of central and peripheral physiologic actions. It seemed to be involved in vasoconstriction and vasodilation, regulation of body temperature, sleep, and hormonal regulation, and evidence suggested that it might be involved in depression. The structural similarity between 5-HT and the then recently discovered hallucinogenic agent (+)-lysergic acid diethylamide (LSD) intrigued investigators. This observation led to speculation that 5-HT might be involved in the mechanism of action of psychoactive substances and that it might play a seminal role in various mental disorders. |
|
Uses |
neurotransmitter |
|
Definition |
ChEBI: A primary amino compound that is the 5-hydroxy derivative of tryptamine. |
|
Biological Functions |
Serotonin (5-hydroxytryptamine, or 5HT) is present in the brain as well as in the periphery. In humans, about 90% of the total serotonin in the body is in enterochromaffin cells in the gastrointestinal tract; the remaining 10% occurs primarily in the platelets and brain. The physiological significance of the vast amounts of serotonin constantly synthesized and metabolized in the periphery still remains an enigma. Brain serotonin has been implicated as a potential neurotransmitter in the mediation of a wide variety of phenomena. |
|
Clinical Use |
Initially, serotonin was thought to be a sleep-promoting neurotransmitter or an “antiwaking” agent. The recognition of the numerous 5-HT receptor subtypes, often with unique anatomical distribution, has required that a more complex role for serotonin be developed. Current studies indicate that conditions for sleep are now met when the serotoninergic system becomes inactive. The serotonin agonists for the 5-HT1 (via the 5-HT1A and 5-HT1B types at the hypothalamic level), 5-HT2, and 5-HT3 receptors cause wakefulness and inhibit sleep. Blockade of the 5-HT2 receptors (e.g., the 5-HT2 antagonist ritanserin) results in increased NREM sleep and inhibition of REM sleep. It has been proposed that the 5-HT1A and 5- HT2 may be involved in sleep by regulation of sleep-promoting substances in the hypothalamus. With the development of newer and more selective ligands for use in studying the numerous serotonin receptor subtypes, a better understanding of the role of serotonin in sleep will evolve. |
|
Metabolism |
The major route of metabolism for 5-HT is oxidative deamination by monoamine oxidase (MAO-A) to the unstable 5-hydroxyindole- 3-acetaldehyde, which is either reduced to 5-hydroxytryptophol (~15%) or oxidized to 5-hydroxyindole-3-acetic acid (~85%). In the pineal gland, 5-HT is acetylated by 5-HT N-acetyltransferase to N-acetylserotonin, which undergoes O-methylation by 5-hydroxyindole-O-methyltransferase to melatonin. |
InChI:InChI=1/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2
50-67-9 Relevant articles
-
Gryglewski et al.
, p. 471,472 (1966)
-
A Visible-Light-Sensitive Caged Serotonin
Cabrera, Ricardo,Filevich, Oscar,García-Acosta, Beatriz,Athilingam, Jegath,Bender, Kevin J.,Poskanzer, Kira E.,Etchenique, Roberto
, p. 1036 - 1042 (2017)
Serotonin, or 5-hydroxytryptamine (5HT),...
Studies of enzyme-mediated reactions. Part 161. Stereochemical course of the formation of 5-hydroxytryptamine (serotonin) by decarboxylation of (2S)-5-hydroxytryptophan with the aromatic L-amino acid decarboxylase (E.C. 4.1.1.28) from Hog Kidney
Battersby,Scott,Staunton
, p. 4685 - 4696 (1990)
-
Conversion of 5-hydroxytryptophan into serotonin by tryptophan decarboxylase in plants, Escherichia coli, and yeast
Park, Munyoung,Kang, Kiyoon,Park, Sangkyu,Back, Kyoungwhan
, p. 2456 - 2458 (2008)
The L-tryptophan decarboxylase (TDC) gen...
Rice histone deacetylase 10 and Arabidopsis histone deacetylase 14 genes encode N-acetylserotonin deacetylase, which catalyzes conversion of N-acetylserotonin into serotonin, a reverse reaction for melatonin biosynthesis in plants
Lee, Kyungjin,Lee, Hyoung Yool,Back, Kyoungwhan
, (2018)
In plants, melatonin production is stric...
Investigation of a substrate-specifying residue within Papaver somniferum and Catharanthus roseus aromatic amino acid decarboxylases
Torrens-Spence, Michael P.,Lazear, Michael,Von Guggenberg, Renee,Ding, Haizhen,Li, Jianyong
, p. 37 - 43 (2014)
Plant aromatic amino acid decarboxylases...
-
Petrova et al.
, (1971)
-
CONVERSION OF TRYPTAMINE TO SEROTONIN BY CELL SUSPENSION CULTURES OF PEGANUM HARMALA
Courtois, Didier,Yvernel, Daniel,Florin, Bruno,Petiard, Vincent
, p. 3137 - 3142 (1988)
Biotransformation of tryptamine to serot...
Sekiguchi lesion gene encodes a cytochrome P450 monooxygenase that catalyzes conversion of tryptamine to serotonin in rice
Fujiwara, Tadashi,Maisonneuve, Sylvie,Isshiki, Masayuki,Mizutani, Masaharu,Chen, Letian,Ling Wong, Hann,Kawasaki, Tsutomu,Shimamoto, Ko
, p. 11308 - 11313 (2010)
Serotonin is a well known neurotransmitt...
Paliperidone Reversion of Maternal Immune Activation-Induced Changes on Brain Serotonin and Kynurenine Pathways
MacDowell, Karina S.,Munarriz-Cuezva, Eva,Meana, J. Javier,Leza, Juan C.,Ortega, Jorge E.
, (2021/06/07)
Emerging evidence indicates that early-l...
The Study of Stability of Proline-Containing Derivatives of Dopamine and Serotonin in the Biological Media in Vitro Experiments
Andreeva, L. A.,Myasoedov, N. F.,Nagaev, I. Yu.,Shevchenko, K. V.,Shevchenko, V. P.
, p. 150 - 158 (2020/05/28)
Abstract—: The peptides Boc-Gly-Pro-DP, ...
Facile in Vitro Biocatalytic Production of Diverse Tryptamines
McDonald, Allwin D.,Perkins, Lydia J.,Buller, Andrew R.
, p. 1939 - 1944 (2019/07/08)
Tryptamines are a medicinally important ...
Biocatalytic Production of Psilocybin and Derivatives in Tryptophan Synthase-Enhanced Reactions
Blei, Felix,Baldeweg, Florian,Fricke, Janis,Hoffmeister, Dirk
, p. 10028 - 10031 (2018/07/29)
Psilocybin (4-phosphoryloxy-N,N-dimethyl...
50-67-9 Process route
-
- 147918-24-9
N-Benzyl-2-<5-(benzyloxy)-3-indolyl>-1-ethanamin

-
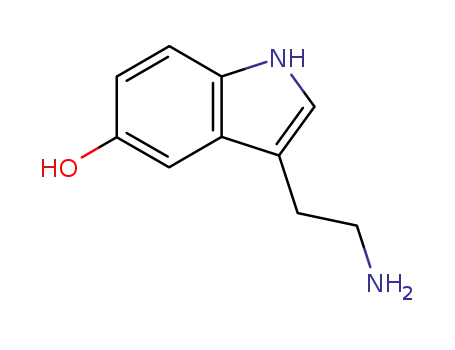
- 50-67-9
3-(2-aminoethyl)-1H-indol-5-ol
| Conditions | Yield |
|---|---|
|
With palladium 10% on activated carbon; ammonium formate; In methanol; at 70 ℃; for 0.75h; Inert atmosphere;
|
100% |
-
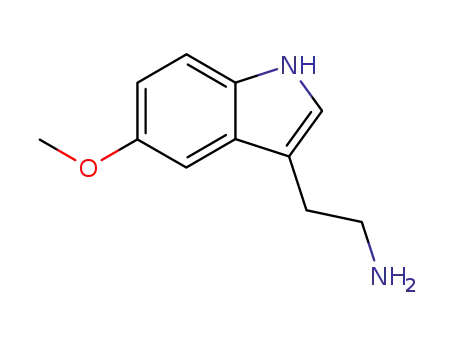
- 608-07-1
2-(5-methoxyindol-3-yl)ethylamine

-

- 50-67-9
3-(2-aminoethyl)-1H-indol-5-ol
| Conditions | Yield |
|---|---|
|
With boron tribromide; In dichloromethane; at 20 ℃;
|
65% |
|
With aluminium trichloride; benzene;
|
50-67-9 Upstream products
-
608-07-1

2-(5-methoxyindol-3-yl)ethylamine
-
20776-45-8
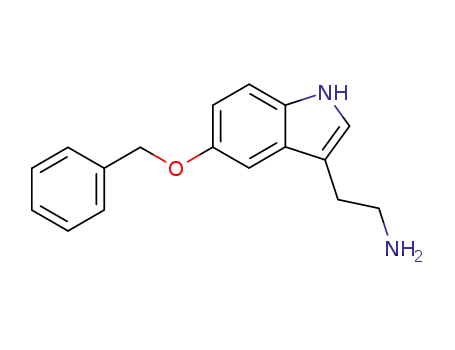
5-benzyloxytryptamine
-
93331-75-0
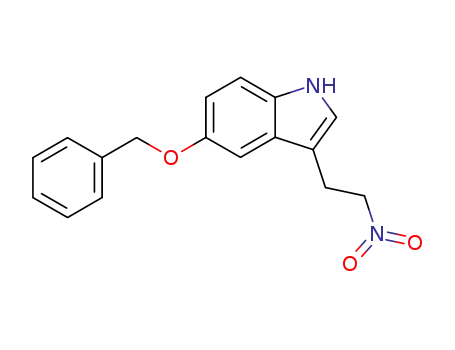
5-benzyloxy-3-(2-nitro-ethyl)-indole
-
55895-70-0
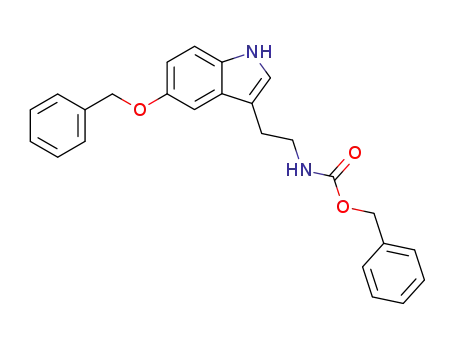
[2-(5-benzyloxy-indol-3-yl)-ethyl]-carbamic acid benzyl ester
50-67-9 Downstream products
-
17994-22-8
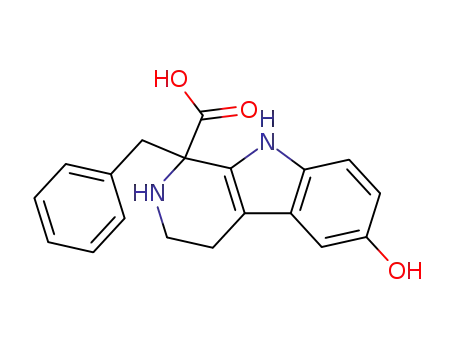
1-benzyl-1,2,3,4-tetrahydro-8-hydroxy-β-carboline-1-carboxylic acid
-
102250-06-6
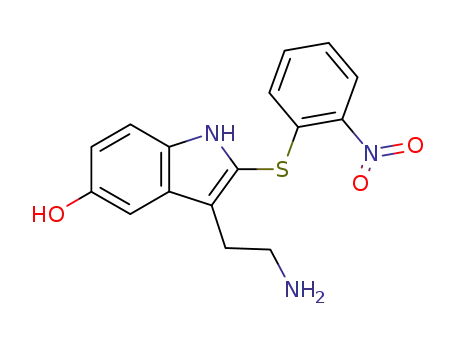
3-(2-Amino-ethyl)-2-(2-nitro-phenylsulfanyl)-1H-indol-5-ol
-
102250-04-4
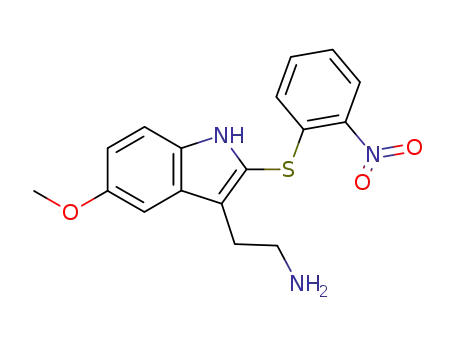
2-[5-Methoxy-2-(2-nitro-phenylsulfanyl)-1H-indol-3-yl]-ethylamine
-
102265-54-3
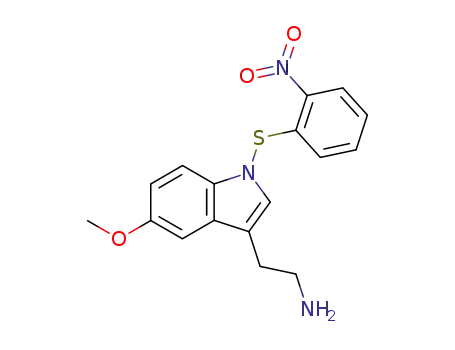
2-[5-Methoxy-1-(2-nitro-phenylsulfanyl)-1H-indol-3-yl]-ethylamine
Relevant Products
-
Corticotropin
CAS:9002-60-2
-
V-9-M cholecystokinin nonapeptide
CAS:99291-20-0