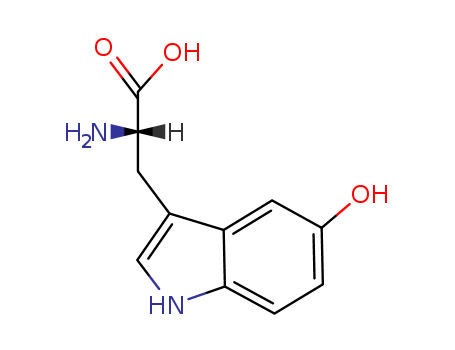
4350-09-8
- Product Name:L-5-Hydroxytryptophan/5-Hydroxytryptophan (5-HTP)/oxitriptan
- Molecular Formula:C11H12N2O3
- Purity:99%
- Molecular Weight:220.228
Product Details;
CasNo: 4350-09-8
Molecular Formula: C11H12N2O3
Appearance: white to pale grey powder
Reputable Factory Supply High Quality L-5-Hydroxytryptophan/5-Hydroxytryptophan (5-HTP)/oxitriptan 4350-09-8 with Competitive Price
- Molecular Formula:C11H12N2O3
- Molecular Weight:220.228
- Appearance/Colour:white to pale grey powder
- Melting Point:270 °C (dec.)(lit.)
- Refractive Index:1.4850
- Boiling Point:520.6 °C at 760 mmHg
- PKA:2.22±0.10(Predicted)
- Flash Point:268.7 °C
- PSA:99.34000
- Density:1.484 g/cm3
- LogP:1.52820
L-5-Hydroxytryptophan(Cas 4350-09-8) Usage
|
Chemical Properties |
white to pale grey powder |
|
Uses |
5-Hydroxy-L-Tryptophan is a hydroxylated metabolite of L-Tryptophan |
|
General Description |
Colorless to pale pink crystals. |
|
Air & Water Reactions |
L-5-Hydroxytryptophan is sensitive to air. Slightly water soluble . |
|
Reactivity Profile |
L-5-Hydroxytryptophan reacts with bases. . |
|
Fire Hazard |
Flash point data for L-5-Hydroxytryptophan are not available, however, L-5-Hydroxytryptophan is probably combustible. |
|
Biochem/physiol Actions |
Hydroxylation of L-tryptophan occurs in the brain during serotonin synthesis. This is a step that controls the production of serotonin and also yields 5-hydroxy-l-tryptophan (5-HTP) as a metabolite. Thus, consumption 5-HTP, increases the serotonin level, which might result in depression, lack of sleep and severe headache. |
|
Purification Methods |
Likely impurities are 5-hydroxy-D-tryptophan and 5-benzyloxytryptophan. Crystallise 5-hydroxy-L-tryptophan under nitrogen from water by adding EtOH. Store it under nitrogen. Also dissolve it in the minimum volume of hot H2O (~0.7g in 4mL) under nitrogen (charcoal) and allowed it to crystallise at 5o. The picrolonate crystallises from H2O with m 184-186o(dec). [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 2732-2737 1961, Morris & Armstrong J Org Chem 22 306 1957, Beilstein 22/14 V 278.] |
InChI:InChI=1/C11H12N2O3/c12-9(11(15)16)3-6-5-13-10-2-1-7(14)4-8(6)10/h1-2,4-5,9,13-14H,3,12H2,(H,15,16)
4350-09-8 Relevant articles
Synthesis of redox-active fluorinated 5-hydroxytryptophans as molecular reporters for biological electron transfer
Ohler, Amanda,Long, Hanna,Ohgo, Kei,Tyson, Kristin,Murray, David,Davis, Amanda,Whittington, Chris,Hvastkovs, Eli G.,Duffy, Liam,Haddy, Alice,Sargent, Andrew L.,Allen, William E.,Offenbacher, Adam R.
supporting information, p. 3107 - 3110 (2021/04/02)
Fluorinated 5-hydroxytryptophans (Fn-5HO...
Biocatalysts from cyanobacterial hapalindole pathway afford antivirulent isonitriles against MRSA
Bunn, Brittney M,Xu, Mizhi,Webb, Chase M,Viswanathan, Rajesh
, (2021/04/26)
Abstract: The emergence of resistance to...
Biocatalytic Production of Psilocybin and Derivatives in Tryptophan Synthase-Enhanced Reactions
Blei, Felix,Baldeweg, Florian,Fricke, Janis,Hoffmeister, Dirk
, p. 10028 - 10031 (2018/07/29)
Psilocybin (4-phosphoryloxy-N,N-dimethyl...
Mutagenesis of an Active-Site Loop in Tryptophan Hydroxylase Dramatically Slows the Formation of an Early Intermediate in Catalysis
Subedi, Bishnu P.,Fitzpatrick, Paul F.
supporting information, p. 5185 - 5192 (2018/04/23)
Solution studies of the aromatic amino a...
4350-09-8 Process route
-
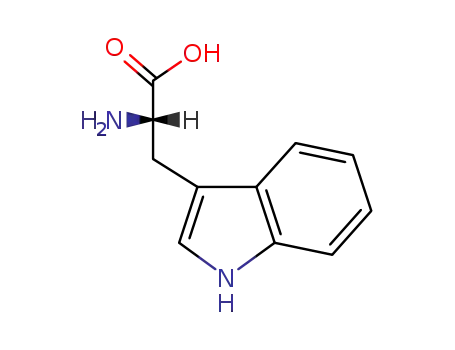
- 73-22-3,27732-43-0,80206-30-0,27813-82-7
L-Tryptophan

-
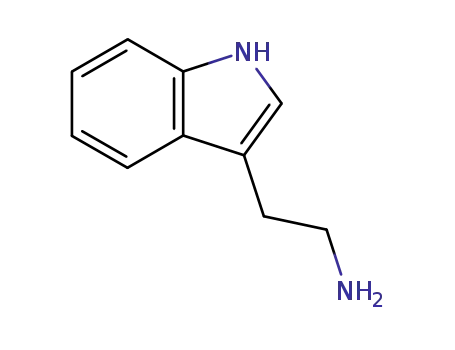
- 61-54-1
tryptamine

-
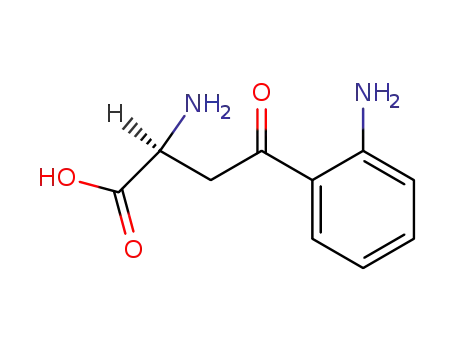
- 2922-83-0
L-kynurenine

-
![hydroxyhexahydropyrrolo[2,3-b]indole-2-carboxylic acid](/upload/2024/4/a1bed1a4-016c-4f29-86e2-ecb143faa678.png)
-
hydroxyhexahydropyrrolo[2,3-b]indole-2-carboxylic acid

-
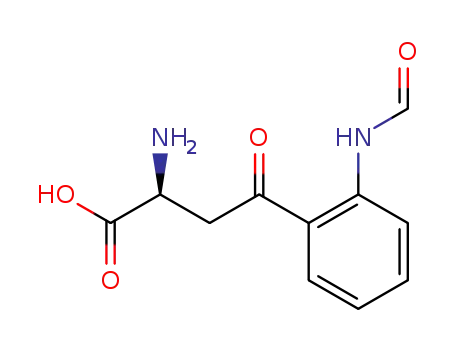
- 3978-11-8
(S )-2-Amino-4-(2-formamidophenyl)-4-oxobutanoic acid

-
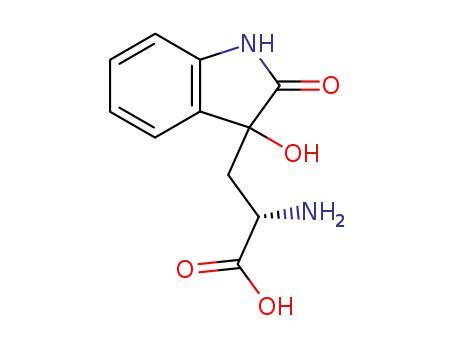
- 184955-21-3
hydroxyoxoindolylalanine

-
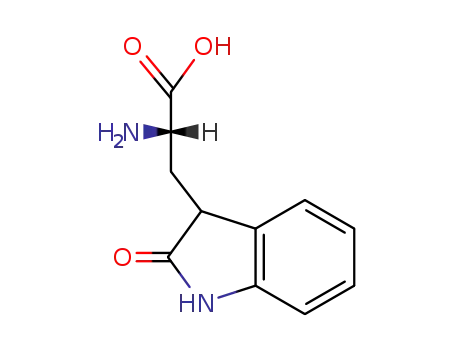
- 236094-74-9
2-amino-3-(2-oxoindolin-3-yl)propanoic acid

-
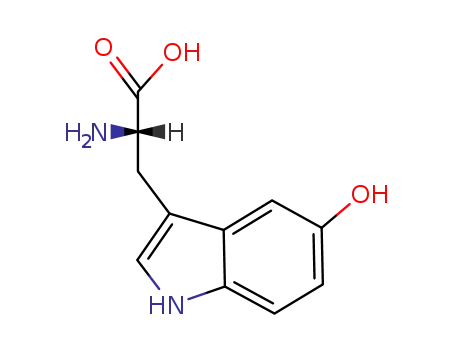
- 4350-09-8,4298-20-8
L-5-HTP
| Conditions | Yield |
|---|---|
|
With dihydrogen peroxide; at 37 ℃; for 6h; pH=4.6; Darkness; aq. borate buffer;
|
-
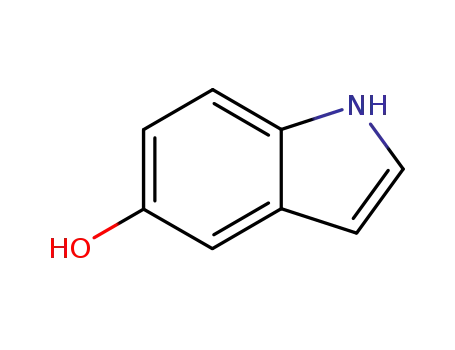
- 1953-54-4
indol-5-ol

-
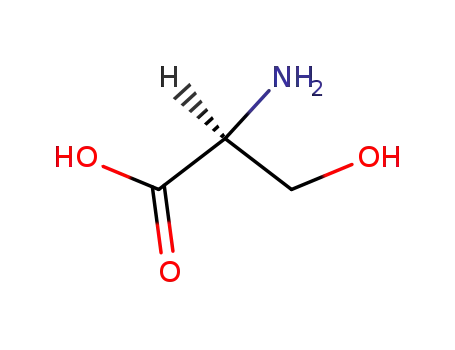
- 56-45-1,83245-15-2
L-serin

-

- 4350-09-8,4298-20-8
L-5-HTP
| Conditions | Yield |
|---|---|
|
indol-5-ol; L-serin; With pyridoxal 5'-phosphate; thermotoga maritima with tryptophan synthase β-subunit from pyrococcus furiosus M145T N167D mutant; In aq. phosphate buffer; water; dimethyl sulfoxide; at 75 ℃; for 2h; pH=8; Sealed tube; Enzymatic reaction;
With hydrogenchloride; In water;
|
93% |
|
With transformed Escherichia coli cells; at 37 ℃; for 24h;
|
70 % Chromat. |
|
With tryptophan synthase; pyridoxal 5'-phosphate; at 37 ℃; for 24h; pH=7.8; Enzymatic reaction;
|
|
|
With Psilocybe cubensis enzymes TrpB; In aq. buffer; at 25 ℃; for 4h; pH=8; Enzymatic reaction;
|
|
|
With Thermotoga maritima pyridoxal phosphate (PLP)-dependent tryptophan B synthase; In aq. phosphate buffer; at 37 ℃; for 4h; pH=7; Enzymatic reaction;
|
|
|
With Thermotoga maritima tryptophan synthase; at 80 ℃; for 48h; Green chemistry; Enzymatic reaction;
|
4350-09-8 Upstream products
-
3520-59-0
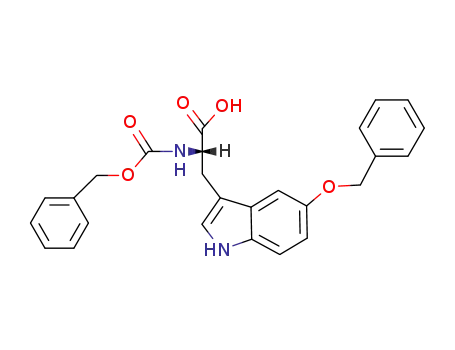
L-N-benzyloxycarbonyl-5-benzyloxytryptophan
-
1953-54-4

indol-5-ol
-
56-45-1

L-serin
-
73-22-3

L-Tryptophan
4350-09-8 Downstream products
-
50-67-9
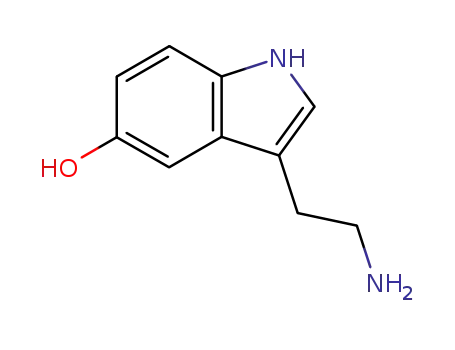
3-(2-aminoethyl)-1H-indol-5-ol
-
132185-16-1
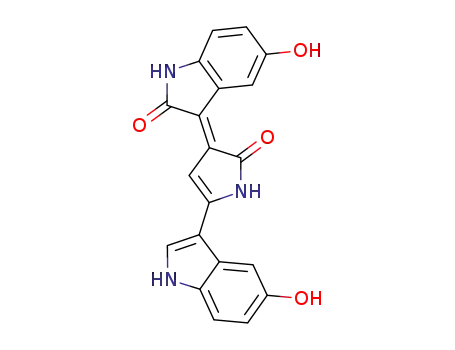
Oxyviolacein
-
548-54-9
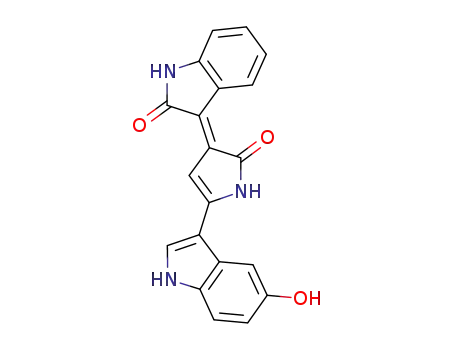
violacein
-
5839-61-2
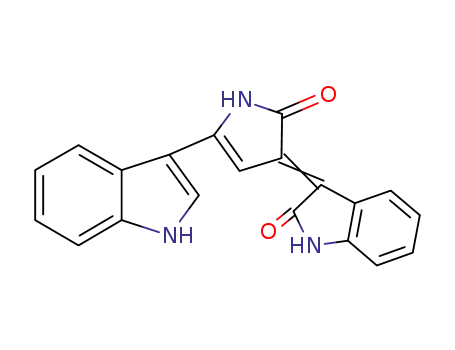
Deoxyviolacein
Relevant Products
-
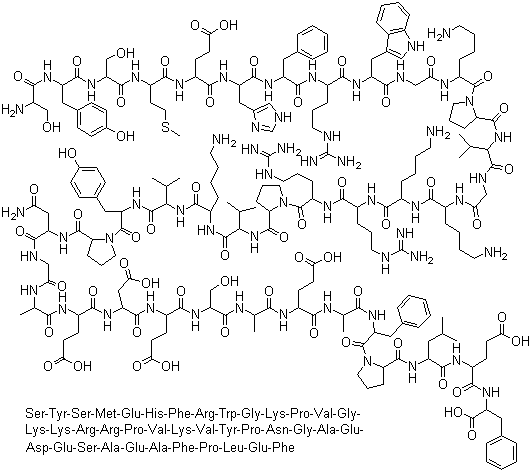
Corticotropin
CAS:9002-60-2
-
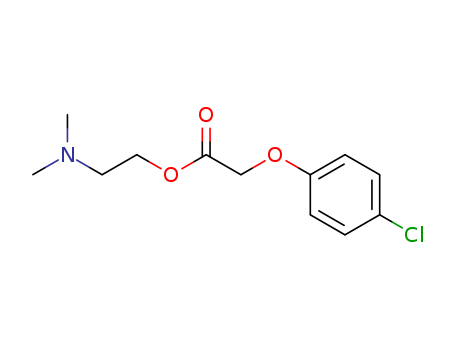
2-(Dimethylamino)ethyl (4-chlorphenoxy)acetate
CAS:51-68-3
-
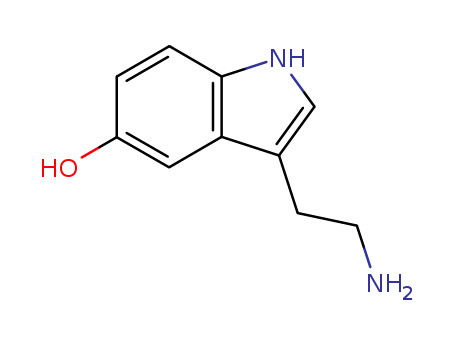
5-Hydroxytryptamine(5-HT)
CAS:50-67-9