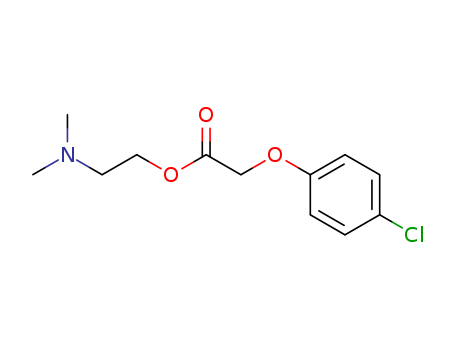
51-68-3
- Product Name:2-(Dimethylamino)ethyl (4-chlorphenoxy)acetate
- Molecular Formula:C12H16ClNO3
- Purity:99%
- Molecular Weight:257.717
Product Details;
CasNo: 51-68-3
Molecular Formula: C12H16ClNO3
Reliable Factory Supply Quality 2-(Dimethylamino)ethyl (4-chlorphenoxy)acetate 51-68-3 with Safe Shipping
- Molecular Formula:C12H16ClNO3
- Molecular Weight:257.717
- Vapor Pressure:5.95E-05mmHg at 25°C
- Melting Point:138-140 °C
- Refractive Index:1.52
- Boiling Point:345.941 °C at 760 mmHg
- PKA:8.17±0.28(Predicted)
- Flash Point:163.019 °C
- PSA:38.77000
- Density:1.179 g/cm3
- LogP:1.82360
2-(Dimethylamino)ethyl (4-chlorphenoxy)acetate(Cas 51-68-3) Usage
|
Use Description |
2-(Dimethylamino)ethyl (4-chlorophenoxy)acetate is a chemical compound with diverse applications in different fields. In the agrochemical industry, it serves as a key ingredient in the formulation of herbicides, contributing to weed control in agriculture. Its role is vital in protecting crops and improving agricultural yields. Additionally, in the pharmaceutical sector, this compound can be employed as an intermediate in the synthesis of pharmaceuticals and organic molecules, offering versatility in drug discovery and development. Moreover, in academic research and organic chemistry, it functions as a building block for the creation of complex molecules, facilitating studies in chemical synthesis and the exploration of new chemical reactions. Its multifaceted applications highlight its significance in agriculture, pharmaceuticals, and chemical research, contributing to crop protection, drug development, and the advancement of chemical science. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C12H16ClNO3/c1-14(2)7-8-16-12(15)9-17-11-5-3-10(13)4-6-11/h3-6H,7-9H2,1-2H3
51-68-3 Relevant articles
Chlorination Reaction of Aromatic Compounds and Unsaturated Carbon-Carbon Bonds with Chlorine on Demand
Liu, Feng,Wu, Na,Cheng, Xu
supporting information, p. 3015 - 3020 (2021/05/05)
Chlorination with chlorine is straightfo...
Esterquat herbicidal ionic liquids (HILs) with two different herbicides: Evaluation of activity and phytotoxicity
Syguda, Anna,Gielnik, Anna,Borkowski, Andrzej,Wo?niak-Karczewska, Marta,Parus, Anna,Piechalak, Aneta,Olejnik, Anna,Marecik, Roman,?awniczak, ?ukasz,Chrzanowski, ?ukasz
supporting information, p. 9819 - 9827 (2018/06/18)
Herbicidal ionic liquids (HILs) constitu...
POTENTIAL CEREBRAL STIMULANTS: ESTERS OF 2-DIMETHYLAMINOETHANOL WITH SOME LIPOPHILIC CARBOXYLIC ACIDS
Protiva, Miroslav,Valenta, Vladimir,Kopicova, Zdenka,Lukac, Juraj,Holubek, Jiri,Krejci, Ivan
, p. 1278 - 1289 (2007/10/02)
2-Dimethylaminoethyl esters of 2,2-dimet...
51-68-3 Process route
-
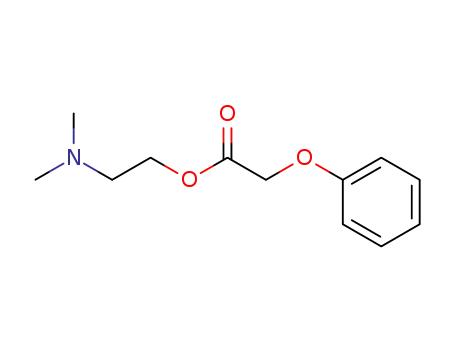
- 50837-15-5
2-(dimethylamino)ethyl phenoxyacetate

-
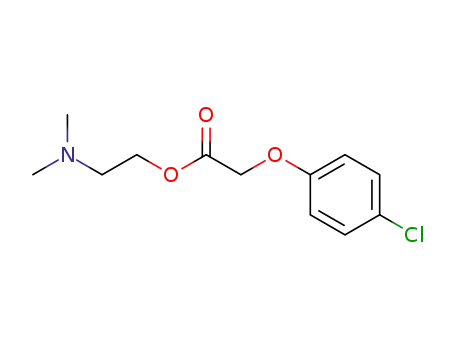
- 51-68-3
meclofenoxate
| Conditions | Yield |
|---|---|
|
With tetraethylammonium chloride; trichloroacetonitrile; In acetonitrile; at 23 ℃; for 2h; Electrochemical reaction; Inert atmosphere;
|
52% |
-
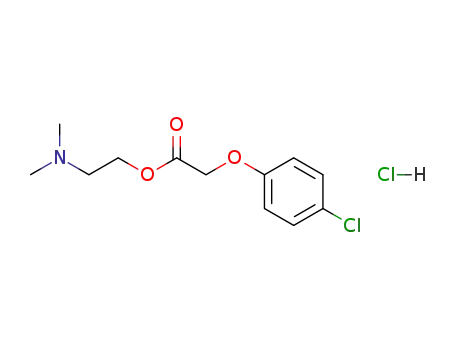
- 3685-84-5
meclofenoxate hydrochloride

-

- 51-68-3
meclofenoxate
| Conditions | Yield |
|---|---|
|
With triethylamine; In chloroform;
|
78% |
51-68-3 Upstream products
-
108-01-0
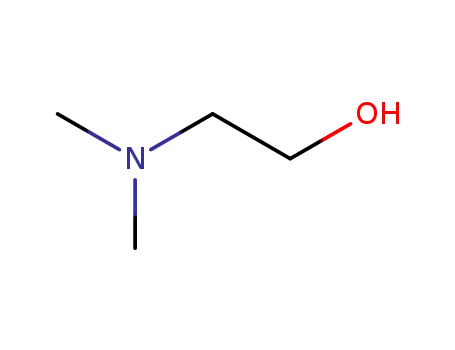
2-(N,N-dimethylamino)ethanol
-
4122-68-3
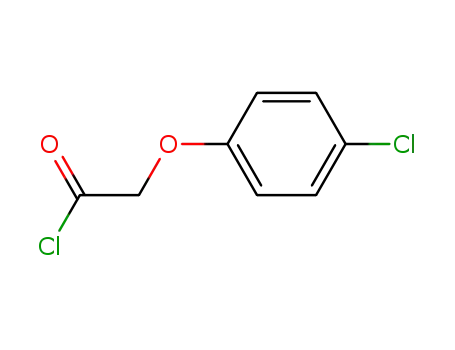
4-chlorophenyloxyacetyl chloride
-
24101-91-5
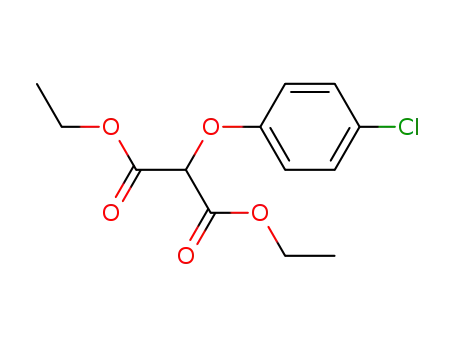
2-(4-chlorophenoxy)-1,3-propanedioic acid diethyl ester
-
2130-69-0
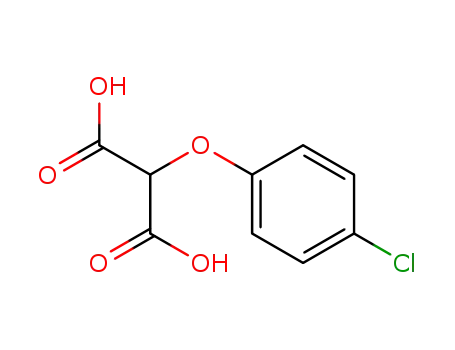
(4-chlorophenoxy)malonic acid
51-68-3 Downstream products
-
4841-22-9
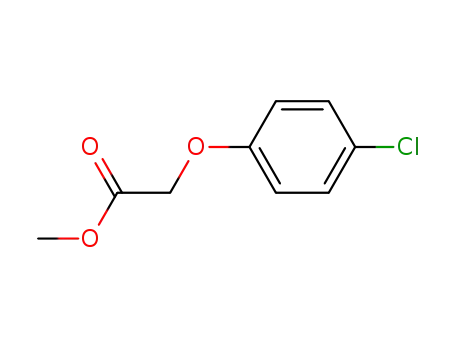
(4-chloro-phenoxy)-acetic acid methyl ester
-
122-88-3
4-Chlorophenoxyacetic acid
Relevant Products
-
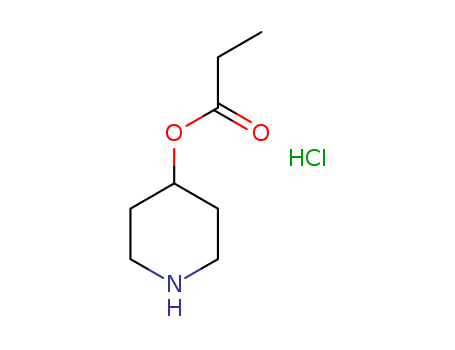
4-PIPERIDINOL, PROPIONATE, HYDROCHLORIDE
CAS:219859-83-3
-
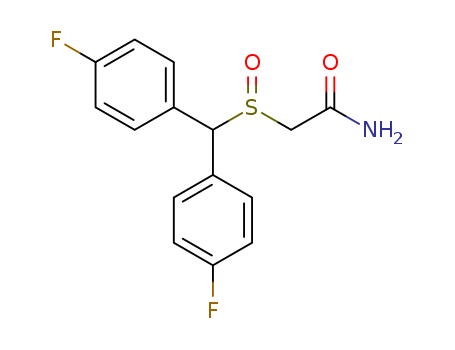
BisfluoroModafinil
CAS:90280-13-0